| Aladdin Scientific | |
|---|---|
| Country: | United States |
| Tel: | 400-620-6333 |
| E-mail: | sales@aladdinsci.com |
| QQ: | |
| Skype: | Chat Now! |
【Aladdin】Immunoprecipitation (IP) lysates and reagents
Release time: 2024-03-29
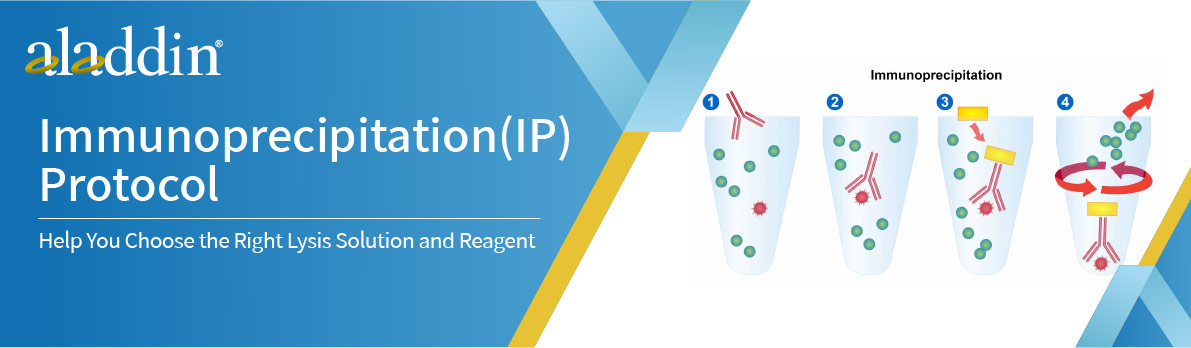
Immunoprecipitation is a protein purification method. An antibody for the protein of interest is incubated with a cell extract, allowing it to bind to the protein in solution. The antibody/antigen complex is then isolated from the sample using protein A/G-coupled agarose beads. This separates the protein of interest from the rest of the sample, which can then be analyzed by SDS-PAGE for western blotting.
Lysis buffers
The lysis buffer should release sufficient proteins from the sample while minimizing protein denaturation. Non-ionic detergents, such as NP-40 and Triton X-100, are less harsh than ionic detergents, such as SDS and sodium deoxycholate. Other variables that can affect the success of immunoprecipitation include salt concentration, divalent cation concentration, and pH. To optimize these variables, they should be tested within the ranges.
-Salts: 0-1 M
-Detergent, non-ionic: 0.1-2%
-Detergent, ionic: 0.01-0.5%
-Divalent cations: 0-10 mM
-EDTA: 0-5 mM
-pH: 6-9
Non-denaturing lysis buffer
Use this for antigens that are soluble in detergents and are recognized in their native form by the antibody. Triton X-100 can be used as a substitute for NP-40.
-20 mM Tris HCl pH 8
-137 mM NaCl
-1% Nonidet P-40 (NP-40)
-2 mM EDTA
Store up to 6 months at 4°C. Immediately before use add protease inhibitors.
For convenience, a 10% sodium deoxycholate stock solution (5g into 50mL) may be prepared. It must be protected from light.
Detergent-free soluble protein lysis buffer
Certain soluble proteins may not require the use of detergents. This buffer is suitable for mechanical cell lysis, such as homogenization with a Dounce homogenizer.
PBS containing:
-5 mM EDTA
-0.02% sodium azide
Store up to 6 months at 4°C. Immediately before use add protease inhibitors.
Denaturing lysis buffer for non-detergent soluble antigens
Epitopes of native proteins are not accessible to antibodies that only recognise denatured proteins. To harvest and lyse the cells, heat them in denaturing lysis buffer. This method can also be used for antigens that cannot be extracted from the cell with non-ionic detergents. The use of DNase1 will aid in the extraction of proteins from chromatin.
-1% SDS
-5 mM EDTA
Store up to 1 week at room temperature.
Immediately before use add
-10 mM dithiothreitol or beta-mercaptoethanol
-Protease inhibitors15 U/mL DNase1
Wash buffers
-10mM Tris; adjust to pH 7.4
-1mM EDTA
-1mM EGTA; pH 8.0
-150mM NaCl
-1% Triton X-100
-0.2mM sodium orthovanadate
Store up to 6 months at 4°C. Immediately before use add protease inhibitor.
Other reagents
Protease inhibitors
Proteolysis, dephosphorylation, and denaturation start immediately after cell lysis. To slow down these processes, samples should be placed on ice. Additionally, protease and phosphatase inhibitor cocktails can be used. If a cocktail is not available, PMSF (50 μg/mL) and aprotinin (1 μg/mL) are commonly used protease inhibitors for immunoprecipitation.
Other reagents
-Sterile PBS pH 7.4
-Sterile PBS-BSA 1% w/v (filtered)
-TBST buffer
-Loading/sample buffer for western blotting
-VeriBlot for immunoprecipitation secondary antibodies, which preferentially detect the non-reduced, non-denatured primary antibody during western blotting.
-100mM EDTA stock solution is made with 1.86g EDTA dissolved into 40mL H2O. Add NaOH to adjust the pH to7.4. Finally, adjust the total volume to 50mL.
Preparing the lysates
Lysates from cell culture (non-denaturing)
1,Place the cell culture dish on ice and wash the cells with ice-cold PBS.
2,Drain the PBS, then add ice-cold lysis buffer (1mL per 107 cells/100 mm2 dish/150 cm2 flask; 0.5 mL per 5×106cells/60 mm2 dish or 75cm2 flask).
3,Scrape adherent cells from the dish using a cold plastic cell scraper and gently transfer the cell suspension to a pre-cooled microcentrifuge tube.
4,Stir continuously for 30 min at 4°C.
5,Centrifuge in a microcentrifuge at 4°C.
You may need to vary the centrifugation force and time depending on the cell type. A guideline is 20 minutes at 12,000 rpm, but you should optimize this for your specific experiment (e.g. leucocytes need very light centrifugation).
6,Carefully remove the tubes from the centrifuge and place on ice. Aspirate the supernatant, transfer to a fresh tube on ice and discard the pellet.
Lysates from cell culture (denaturing)
1,Add 100 μL denaturing lysis buffer to 0.5–2×107 cells.
2,Mix well by vertexing vigorously for 2-3 seconds at maximum speed. Transfer the cell suspension to a micro-centrifuge tube.
The solution may be viscous at this stage due to the release of DNA.
3,Heat samples to 95°C for 5 min to denature.
4,Dilute the suspension with 0.9 mL non-denaturing lysis buffer and mix gently.
An excess of 1% Triton X-100 in non-denaturing lysis buffer quenches SDS in the original denaturing buffer.
5,Fragment the DNA by passing the lysed suspension 5-10 times through a needle attached to a 1ml syringe.
Repeat the mechanical disruption until the viscosity is reduced to a manageable level. If the DNA is not completely digested and fragmented, it may interfere with the separation of the pellet and supernatant after centrifugation.
6,Incubate on ice for 5mins.
7,Proceed with the immunoprecipitation
Lysates from tissue
1,Dissect the tissue with clean instruments as quickly as possible. If possible, do this on ice to prevent protease degradation.
2,Place the tissue in round-bottomed micro-centrifuge tubes and immerse in liquid nitrogen to snap freeze. Store samples at -80°C for later use or on ice for immediate homogenization.
3,For a ~5mg piece of tissue, rapidly add ~300μL lysis buffer to the tube and homogenize using an electric homogenizer.
4,Rinse the blade twice with a further 300 μL of lysis buffer per wash and agitate for 2 hours at 4°C (e.g. on an orbital shaker in a refrigerator).
Lysis buffer volumes must be determined in relation to the amount of tissue present. Protein extract should not be over-diluted to avoid loss of protein and to minimize the volume of sample to be loaded on the gels. The minimum concentration is 0.1 mg/mL and the optimum concentration is 1-5 mg/mL.
If denatured samples are required, use Denaturing Lysis Buffer and follow steps 2-5 of the Denaturation Protocol above.
5,Centrifuge at 12,000 rpm for 20 minutes at 4°C in a micro-centrifuge. Carefully remove the tubes from the centrifuge, place on ice, aspirate the supernatant and transfer to a fresh tube on ice. Discard the pellet.
Pre-clearing the lysates
Pre-clearing the lysate can help to reduce non-specific binding and background. However, if the final detection of the protein is by Western blotting, pre-clearing may not be necessary unless a contaminating protein interferes with the visualization of the protein of interest.
1,Add either 50 µL of off-target antibody of the same species and isotype as the immunoprecipitation antibody or normal serum (rabbit is often preferred) to 1 mL of lysate. Incubate for 1 hour on ice.
2,Add 100 μL of bead slurry to the lysate.
3,Incubate for 10–30 min at 4°C with gentle agitation.
4,Spin in microcentrifuge at 14,000 g at 4°C for 10 min.
5,Discard bead pellet and keep supernatant for immunoprecipitation.
To increase the yield, the beads can be washed 1 or 2 more times in lysis buffer and the supernatants collected together.
It is important to ensure that as much normal serum as possible is removed as this will compete with the antibody against the antigen of interest. To check this, a test can be run using lysis buffer instead of sample, following all the pre-cleaning steps above.
Running a gel of the resulting supernatant and staining with Coomassie will show whether serum Ig has been effectively removed. If serum has not been sufficiently removed, bands at 50 and 25kDa for heavy and light chains will be present; its presence may contribute to weak immunoprecipitation. Consider either reducing the amount of serum or increasing the number of beads incubated with your samples in the pre-cleaning step.
References
1.Bonifacino, Juan S. et al. Current Protocols in Immunology 8.3.1 -8.3.28, New York: John Wiley, 2001.
2.Harlow, Ed, and David Lane. Using Antibodies. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, 1999.
Aladdin: https://www.aladdinsci.com/


