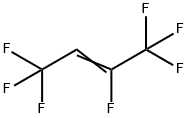
1,1,1,2,4,4,4-HEPTAFLUORO-2-BUTENE synthesis
- Product Name:1,1,1,2,4,4,4-HEPTAFLUORO-2-BUTENE
- CAS Number:760-42-9
- Molecular formula:C4HF7
- Molecular Weight:182.04
87-68-3
146 suppliers
$10.00/1g
400-44-2
30 suppliers
$95.00/5G
303-04-8
3 suppliers
$101.00/10g
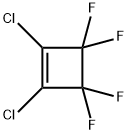
377-93-5
33 suppliers
$45.00/250mg

760-42-9
24 suppliers
inquiry
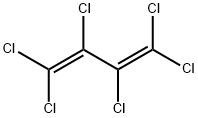
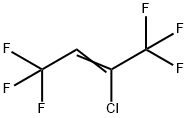
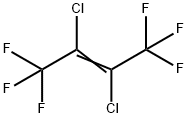
Yield:760-42-9 12% ,303-04-8 29% ,400-44-2 39% ,377-93-5 9%
Reaction Conditions:
with hydrogen fluoride at 380;
Steps:
1.1 The preparation method of 1,1,1,2,4,4,4-heptafluoro-2-butene provided in this embodiment comprises the following steps:
(1) Preparation of chromium-based catalyst precursor: using a coprecipitation method, the steps are as follows,CrCl3, Zn(NO3)2 with a molar ratio of 85:5:10Mix well with the graphene solution and sonicate for 30 minutes at room temperature.30 wt.% of aqueous ammonia was slowly added dropwise to the above mixed solution.Adjust the solution pH=9.0, precipitate the aging after 4 hours, filter the precipitate,The precipitate was washed with deionized water and calcined at 400 ° C to form a press.Thereby a chromium-based catalyst precursor is obtained.Activation of chromium-based catalysts:10 ml of the chromium-based catalyst precursor was charged to the fixed bed reactor.Heating the fixed bed reactor with an open tube heating furnace,Chromium-based catalyst at a nitrogen pressure of 50 ml/min and a temperature of 300 °CDrying for 10 hours; then introducing hydrogen fluoride at a rate of 80 ml/min.Activation of the chromium-based catalyst at 300 ° C for 4 hours,Thereby the activation of the chromium-based catalyst is completed.Synthesis of 1,1,1,2,4,4,4-heptafluoro-2-butene: molar ratio of 1:A mixed gas of hexachlorobutadiene and hydrogen fluoride of 7 was passed through a 380 ° C chromium-based catalyst fixed bed reactor at a rate of 250 ml/min, after that,Through the buffer bottle,Washed bottles, concentrated alkali absorbers, and cooled collectors. After the experiment,The product is mainly distributed in the cooling collector.The collected product was subjected to GC analysis.GC results show that the collected products mainly contain12% 1,1,1,2,4,4,4-heptafluoro-2-butene (molecular formula CF3-CF=CH-CF3),29% dichlorohexafluoro-2-butene (molecular formula CF3-CCl=CCl-CF3),39% 1,1,1,4,4,4-hexafluoro-2-chloro-2-butene (molecular formula CF3-CCl=CH-CF3),9% dichlorotetrafluorocyclobutene (molecular cycle-CF2-CCl=CCl-CF2-cycle).The reaction mixture product was further circulated and fluorinated a plurality of times to finally obtain 1,1,1,2,4,4,4-heptafluoro-2-butene having a purity of 90% or more.
References:
CN108911947,2018,A Location in patent:Paragraph 0027; 0028; 0229; 0030; 0031; 0036; 0041
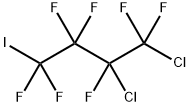
678-13-7
16 suppliers
$25.00/1g
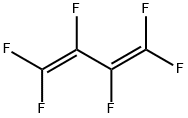
685-63-2
96 suppliers
inquiry

357-21-1
0 suppliers
inquiry
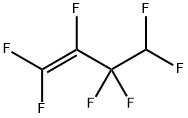
680-54-6
23 suppliers
$60.00/100mg

14671-64-8
0 suppliers
inquiry

760-42-9
24 suppliers
inquiry
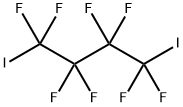
375-50-8
137 suppliers
$25.00/1g
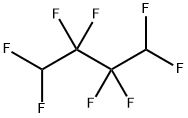
377-36-6
38 suppliers
$25.00/1g

685-63-2
96 suppliers
inquiry

754-73-4
21 suppliers
inquiry

680-54-6
23 suppliers
$60.00/100mg

760-42-9
24 suppliers
inquiry
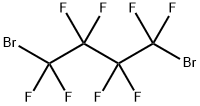
335-48-8
84 suppliers
$26.00/5g
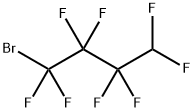
558-86-1
13 suppliers
inquiry

377-36-6
38 suppliers
$25.00/1g

685-63-2
96 suppliers
inquiry

680-54-6
23 suppliers
$60.00/100mg

760-42-9
24 suppliers
inquiry

685-63-2
96 suppliers
inquiry
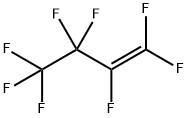
357-26-6
11 suppliers
$195.00/5G
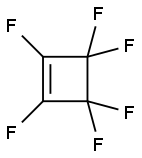
697-11-0
31 suppliers
inquiry

760-42-9
24 suppliers
inquiry
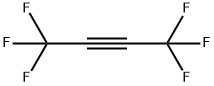
692-50-2
66 suppliers
inquiry