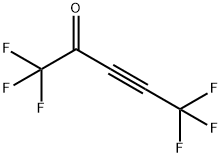
1,1,1,5,5,5-hexafluoropent-3-yn-2-one synthesis
- Product Name:1,1,1,5,5,5-hexafluoropent-3-yn-2-one
- CAS Number:96102-06-6
- Molecular formula:C5F6O
- Molecular Weight:190.04
Yield:96102-06-6 2.6 g
Reaction Conditions:
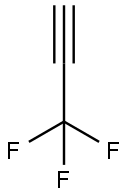
Stage #1: 3,3,3-trifluoroprop-1-ynewith n-butyllithium in n-heptane;tert-butyl methyl ether at -103 - -28; for 0.5 h;Inert atmosphere;Large scale;
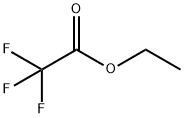
Stage #2: ethyl trifluoroacetate, in n-heptane;tert-butyl methyl ether at -38 - -30; for 2 h;Inert atmosphere;Large scale;Further stages;
Steps:
2-55 Example 2-55
To 36 kg of tert-butyl methyl ether,6.5 kg of 3,3,3-trifluoropropyne(69 mol, 1.0 eq) at -98 to -44 ° C.,Further, an n-heptane solution of n-butyllithium (25 wt%)17 kg (66 mol, 1.0 eq) was added at -103 to -28 ° C., and the mixture was stirred at -30 ° C. for 30 min. further,9.5 kg (67 mol, 1.0 eq) of ethyl trifluoroacetateAt -38 to -33 ° C., and the mixture was stirred at -30 ° C. for 2 hours (reaction completion liquid). The procedure up to here was carried out under a nitrogen gas atmosphere.Sulfuric acid aqueous solution 64 kg [Concentrated sulfuric acid 16 kg (160 mol, 2.4 eq)Prepared from 48 kg of water], the reaction completion solution was added at 3 ° C.,And the mixture was stirred at 5 ° C. for 30 minutes (two-phase system). Two phases were separated and quantified with an internal standard method (internal standard substance; α, α, α-trifluorotoluene) from 19 F-NMR analysis of the recovered organic layer,37 mol of 1,1,1,5,5,5-hexafluoro-3-pentyn-2-nonethylheumiketal,29 mol of a hydrate of 1,1,1,5,5,5-hexafluoro-3-pentyn-2-non was contained. The total yield of the equivalent isIt was quantitative. Preparation of ethylheumiketal and hydrate of 1,1,1,5,5,5-hexafluoro-3-pent-2-19 F-NMR is shown below.To the recovered organic layer obtained, 29 kg of fresh sulfuric acid aqueous solution[Concentrated sulfuric acid 0.29 kg (3.0 mol, 0.045 eq)And 29 kg of water]], and the mixture was stirred at 52 ° C. for 10 hours(Two phase system). Two phases were separated and quantified with an internal standard method (internal standard substance; α, α, α-trifluorotoluene) from 19 F-NMR analysis of the recovered organic layer,1,1,1,5,5,5-hexafluoroacetylacetoneOf dihydrate was contained in an amount of 58 mol. The yield of the target product isIt was 88%. The recovered organic layer was washed with 40 kg of water,Concentrated under reduced pressure (oil bath temperature: ~ 29 ° C, degree of pressure reduction: ~ 6.2 kPa)30 kg of n-heptane was added to the residue, and concentrated under reduced pressure again(Oil bath temperature: ~ 33 ° C, degree of pressure reduction: ~ 5.5 kPa)Further, 45 kg of n-heptane was added, the mixture was stirred at 4 ° C. for 2 hours,The precipitated crystals were filtered and dried under reduced pressure,1,1,1,5,5,5-hexafluoroacetylacetoneOf hydrate (dihydrate: monohydrate = 92: 8) was obtained.The total yield was 82%.4.0 kg (16 mol, 1.0 eq) of the obtained hydrate of 1,1,1,5,5,5-hexafluoroacetylacetone was added to concentrated sulfuric acid 4.0 kg (41 mol, 2.6 eq) at 15 to 20 ° C., and the mixture was stirred at the same temperature for 3 hours and 30 minutes (two-phase system). By separating the dehydrated finished liquid into two phases, 3.3 kg of a crude product of 1,1,1,5,5,5-hexafluoroacetylacetone was obtained. The recovery rate was quantitative. Gas chromatography purity of the crude product was 99.7%. 0.33 kg (3.4 mol, 0.23 eq) of concentrated sulfuric acid was added to 3.2 kg (15 mol, 1.0 eq) of the crude product and subjected to fractional distillation (distillation temperature 69 ° C. / atmospheric pressure) 2.6 kg of the purified product of 1,1,1,5,5,5-hexafluoroacetylacetone shown is obtained. The recovery rate was 81%. The gas chromatography purity of the purified product was 100.0%.
References:
JP2018/90512,2018,A Location in patent:Paragraph 0406; 0409; 0410