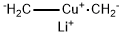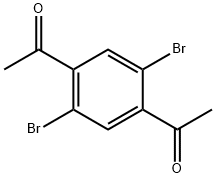
1,1'-(2,5-dibromo-1,4-phenylene)diethanone synthesis
- Product Name:1,1'-(2,5-dibromo-1,4-phenylene)diethanone
- CAS Number:717883-04-0
- Molecular formula:C10H8Br2O2
- Molecular Weight:319.98
Yield:717883-04-0 810 mg
Reaction Conditions:
in tetrahydrofuran at -78; for 3 h;Inert atmosphere;
Steps:
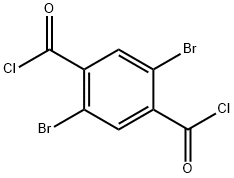
1,1'-(2,5-dibromo-1,4-phenylene)bis(ethan-1-one) (S4)
First, 2,5-dibromoterephthaloyl dichloride was prepared from 2,5-dibromoterephthalic acid: 2,5-dibromoterephthalic acid (2.00 g, 6.17 mmol) was dissolved in dry toluene (30 mL) containing a few drops of dry DMF (~0.5 mL) under N2. Oxalyl dichloride (1.14 mL, 13.3 mmol) was added dropwise to the solution. The mixture was heated to 40 °C in an oil bath and stirred for 16 h. After the reaction time, the solvent was removed under reduced pressure, and the crude product was used without further purification. In parallel, lithium dimethylcuprate(I) was prepared from the reaction of MeLi (19.3 mL, 30.9 mmol, 1.6 M in Et2O) and CuI (4.71 g, 24.7 mmol) in dry THF (30 mL) at -78 °C under N2 atmosphere. The mixture was stirred for 1.5 h while the Gilman reagent precipitated. To the lithium dimethylcuprate(I) slurry, the solution of the as-prepared terephthaloyl dichloride in THF (5 mL) was added slowly at -78 °C under N2. The mixture was stirred for 40 min at -78 °C, and subsequently water (45 mL) was added to the mixture and allowed to warm up to rt. The mixture was filtered through a Celite pad, and the aqueous layer was extracted with Et2O (3x50 mL). The combined organic phase was washed with brine (100 mL) and dried over MgSO4. The solvent was removed under reduced pressure, and the crude product was purified via flash column chromatography (SiO2, hexanes → hexanes/EtOAc (10%)) to afford S4 (810 mg, 41%) as a white solid.
References:
Adorján, áron;Mayer, Péter J.;Szabó, Pál T.;Holczbauer, Tamás;London, Gábor [Synthetic Communications,2022,vol. 52,# 19-20,p. 1956 - 1966] Location in patent:supporting information
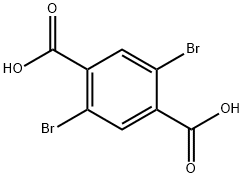
13731-82-3
197 suppliers
$7.00/1g

717883-04-0
14 suppliers
inquiry