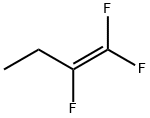
1,1,2-TRIFLUORO-1-BUTENE synthesis
- Product Name:1,1,2-TRIFLUORO-1-BUTENE
- CAS Number:383-84-6
- Molecular formula:C4H5F3
- Molecular Weight:110.08
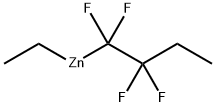
1463983-58-5
0 suppliers
inquiry

383-84-6
24 suppliers
inquiry
Yield:383-84-6 100 %Spectr.
Reaction Conditions:
with lithium iodide in [D3]acetonitrile at 20; for 0.5 h;Inert atmosphere;
Steps:
Reaction of EtCF2CF2ZnEt (5) with LiI
To a CD3CN (0.6 mL) solution of ZnEt2 (10.3 μL, 1.0 M in hexane solution, 0.10 mmol) was added α,α,α-trifluorotoluene as an internal standard. The resultant solution was then transferred into a pressure-tight NMR tube. The NMR tube was degassed and then TFE (3.5 atm, > 0.30 mmol) was charged into the tube. After thermostating the reaction mixture at 60 °C for 8 h, 19F NMR observation revealed that the carbozincation product 5 was generated in 12% yield. The NMR tube was then degassed, and a CD3CN (0.6 mL) solution of LiI (32.6 mg, 0.240 mmol) was added to the resulting solution. 19F NMR observation revealed that 5 was completely converted into 1 in quantitative yield.
References:
Ohashi, Masato;Kamura, Ryohei;Doi, Ryohei;Ogoshi, Sensuke [Chemistry Letters,2013,vol. 42,# 8,p. 933 - 935] Location in patent:supporting information
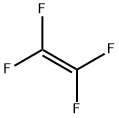
116-14-3
46 suppliers
inquiry
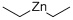
557-20-0
228 suppliers
$14.00/1g

383-84-6
24 suppliers
inquiry

1463983-58-5
0 suppliers
inquiry

