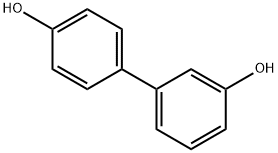
[1,1'-biphenyl]-3,4'-diol synthesis
- Product Name:[1,1'-biphenyl]-3,4'-diol
- CAS Number:18855-13-5
- Molecular formula:C12H10O2
- Molecular Weight:186.21
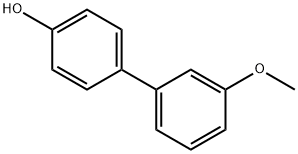
71022-86-1
11 suppliers
inquiry
![[1,1'-biphenyl]-3,4'-diol](/CAS/GIF/18855-13-5.gif)
18855-13-5
17 suppliers
$211.00/1g
Yield:18855-13-5 765 mg
Reaction Conditions:
with boron tribromide in dichloromethane at 20; for 0.5 h;Inert atmosphere;
Steps:
8. 2-(2'-Methoxy-5'-propyl-biphenyl-4-yloxy)-tetrahydro-pyran (10)
To a suspension solution of palladium on carbon (100 mg, 10% by weight) in MeOH (5 mL) was added a solution of 9 (1.0 g, 6.75 mmol) in MeOH (10 mL) and the resulting mixture was stirred under 60 psi hydrogen pressure in the Parr Hydrogenator for 2 h at which time TLC indicated complete disappearance of the starting material. The mixture was filtered to get rid of all the solid materials and after evaporation of the solvent afforded a crude residue. To the solution of the residue (1.0 g, 6.75 mmol) in AcOH (10 mL) was slowly added excess bromine (0.5 mL). The mixture was stirred for 18 h at ambient temperature when TLC indicated total disappearance of the starting material. Solvent was evaporated under vacuum and the residue was extracted with ethyl acetate (20 mL), and the extract was washed thoroughly with saturated Na2S2O3 solution (10 mL), NaHCO3 solution (10 mL) and brine (3 × 10 mL). The organic layer was concentrated under reduced pressure and the crude residue (1.2 g, 5.26 mmol, 78%) was used directly in the next step without further purification. To the solution of crude residue (500 mg, 2.19 mmol) from the previous step and 4 (800 mg, 2.63 mmol) in DME (8 mL) was added the catalyst PdCl2(dppf)·CH2Cl2 (89 mg, 0.109 mmol) under argon atmosphere followed by Na2CO3 solution (3 mL, aq. 2 M) and the mixture was stirred at 80 °C for 18 h. Evaporation of solvent afforded a dark residue which was extracted with EtOAc and the organic extract was repeatedly washed with brine (3 × 20 mL). The organic layer was concentrated under reduced pressure to furnish a dark brown oil which was purified by column chromatography using a mixture of EtOAc and hexanes (1:19) as mobile phase to yield pure 10 (gum, 593 mg, 1.81 mmol, 83%).
References:
Tripathi, Subhankar;Chan, Ming-Huan;Chen, Chinpiao [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 1,p. 216 - 221] Location in patent:supporting information; experimental part

84591-12-8
15 suppliers
$412.00/1g
![[1,1'-biphenyl]-3,4'-diol](/CAS/GIF/18855-13-5.gif)
18855-13-5
17 suppliers
$211.00/1g
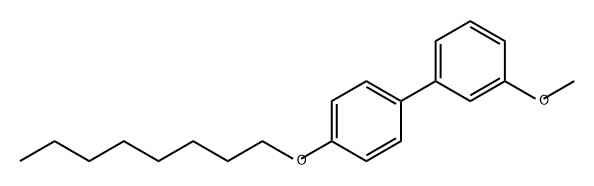
260056-62-0
0 suppliers
inquiry
![[1,1'-biphenyl]-3,4'-diol](/CAS/GIF/18855-13-5.gif)
18855-13-5
17 suppliers
$211.00/1g
![2H-Pyran, 2,2'-[[1,1'-biphenyl]-3,4'-diylbis(oxy)]bis[tetrahydro-](/CAS/20210305/GIF/1287777-07-4.gif)
1287777-07-4
0 suppliers
inquiry
![[1,1'-biphenyl]-3,4'-diol](/CAS/GIF/18855-13-5.gif)
18855-13-5
17 suppliers
$211.00/1g

![Boronic acid, B-[4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]phenyl]-, B,B'-anhydride](/CAS/20240320/GIF/123088-32-4.gif)