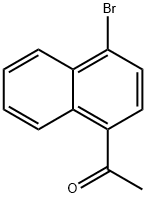
1-(1-BROMONAPHTHALEN-4-YL)ETHANONE synthesis
- Product Name:1-(1-BROMONAPHTHALEN-4-YL)ETHANONE
- CAS Number:46258-62-2
- Molecular formula:C12H9BrO
- Molecular Weight:249.1
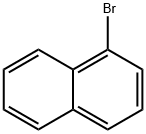
90-11-9
504 suppliers
$5.00/10g

75-36-5
561 suppliers
$17.92/100G

46258-62-2
92 suppliers
$24.00/100mg
Yield: 91%
Reaction Conditions:
with aluminum (III) chloride in 1,2-dichloro-ethane at 0 - 20; for 24 h;
Steps:
3
A solution of 1-Bromo-naphthalene (10 g, 48.3 mmol) and acetyl chloride (4.2 ml, 58 mmol) in 1,2-dichloroethane (100 ml) was cooled to 0°C and aluminum chloride (14.4 g, 108 mmol) was added portion wise. The mixture was stirred at RT for 24 hours. The reaction mixture was poured into ice-water (100 ml). The two layers were separated and the water layer was extracted with diethyl ether (3 x 150 ml). The combined organic layers were dried over magnesium sulfate, filtered and the solvent was removed under reduced pressure to give an orange colored oil. The 1- (4-bromo-naphtalen-1-yl)-ethanone was purified by flash chromatography (cyclohexane/ethylacetate: 95/5), yielding an yellow oil (91% yield). The 1- (4-bromo-naphtalen-1-yl)-ethanone oxime was prepared according to the procedure described for Compound 22, yielding a white powder (98% yield). Activated zinc dust (24.7 g, 379 mmol) was added portion wise to a suspension of the oxime (10.0 g, 37.9 mmol) in acetic acid (40 ml). The mixture was stirred at RT for 2 hours. The zinc dust was removed by filtration and acetic acid was removed under reduced pressure. Water (100 ml) was added and the pH was adjusted to pH = 13 with 1N NaOH. The water layer was extracted with EtOAc (3 x 100 ml). The combined organic layers were dried over MgS04, filtered and the solvent was removed under reduced pressure, yielding a yellow oil (70% yield). Boc20 (7.1 g, 31.8 mmol) was added to a solution of the amine (6.6 g, 26.5 mmol) in 1,4-dioxane (50 ml). The reaction mixture was stirred at RT for 2 hours. The solvent was removed under reduced pressure and the product was purified by flash chromatography (cyclohexane/EtOAc: 95/5), yielding a yellow powder (75% yield). The bromide (350 mg, 1 mmol) was dissolved in THF (13 ml) /water (2 ml). Potassium acetate (100 mg, 1 mmol), 1,3-bis-diphenylphosphinopropane (9.0 mg, 0.02 mmol) and palladium- (11)-acetate (9.0 mg, 0.04 mmol) were added. The mixture was stirred at 50 atm CO pressure and 150°C for 3 hours. The reaction mixture was filtered, the filtrate dried over MgS04 and the solvent was removed under reduced pressure to give a yellow- greenish oil (300 mg). The 4- (l-tert-butoxycarbonylamino-ethyl)-naphthalene-l- carboxylic acid was purified by flash chromatography (DCM/MeOH : 90/10), yielding a white powder (14% yield). The title product was prepared according to the procedure of Compound 31, starting from 4- (1-tert-butoxycarbonylamino-ethyl)-naphthalene-1-carboxylic acid (44 mg) and 4-amino-pyridine (67% yield).'H NMR (300 MHz, , DMSO-d6): 1.64 ppm (d, 3H, J = 6.6 Hz); 5.3 ppm (q, 1H, J = 6.5 Hz), 7.71 ppm (m, 1H), 8.00 ppm (d, 1H, J = 7.7 Hz), 8.32 ppm (m, 1H), 8. 35 ppm (d, 1H, J = 7.3 Hz), 8.81 ppm (d, 2H, J = 7.2 Hz), 12.2 ppm (s, 1H).
References:
DEVGEN NV WO2005/82367, 2005, A1 Location in patent:Page/Page column 64-65
58149-70-5
0 suppliers
inquiry

46258-62-2
92 suppliers
$24.00/100mg
83-53-4
334 suppliers
$10.00/1g
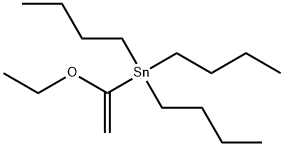
97674-02-7
220 suppliers
$15.00/1 g

46258-62-2
92 suppliers
$24.00/100mg

90-11-9
504 suppliers
$5.00/10g

108-24-7
5 suppliers
$14.00/250ML

46258-62-2
92 suppliers
$24.00/100mg

75-15-0
239 suppliers
$40.00/100 g
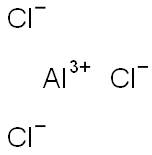
7446-70-0
686 suppliers
$15.00/25g

90-11-9
504 suppliers
$5.00/10g

75-36-5
561 suppliers
$17.92/100G

46258-62-2
92 suppliers
$24.00/100mg