1,1-Di-p-tolyl-2-propyn-1-ol synthesis
- Product Name:1,1-Di-p-tolyl-2-propyn-1-ol
- CAS Number:93318-88-8
- Molecular formula:C17H16O
- Molecular Weight:236.31

611-97-2
238 suppliers
$21.00/25g
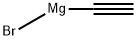
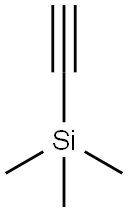
4301-14-8
119 suppliers
$68.50/100ml

93318-88-8
13 suppliers
inquiry
Yield:93318-88-8 85%
Reaction Conditions:
in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
Alkynylation. 1,1-Di(p-tolyl)prop-2-yn-1-ol (1n);25 Typical Procedure.
Procedure A: In a 50 mL oven-dried round-bottomed flask were placed di(p-tolyl) ketone (630 mg, 3 mmol, 1 equiv) and anhyd THF (15 mL) under N2. Ethynylmagnesium bromide (0.5 M in THF, 775 mg, 6 mmol, 2 equiv) was added to the solution at 0 °C and the reaction was allowed to warm up to r.t. After stirring for 3-4 h, the reaction was quenched with sat. aq NH4Cl (5 mL). The resulting mixture was extracted with EtOAc (3 × 10 mL) and the combined organic layers were washed with brine, dried (anhyd MgSO4), filtered, and evaporated. The residue was purified by flash chromatography (silica gel) withPE/EtOAc as eluent to give the propargylic alcohol 1n25 as a yellow oil; yield: 602 mg (85%). 1H NMR (CDCl3, 400 MHz): δ = 7.56 - 7.50 (m, 4 H), 7.18 (d, J = 8.0 Hz,4 H), 2.89 (s, 1 H), 2.83 (s, 1 H), 2.37 (s, 6 H). 13C NMR (CDCl3, 101 MHz): δ = 141.76, 137.59, 129.00, 125.92, 86.70,75.24, 74.07, 21.12.
References:
Yang, Lu;Zeng, Qingle [Synthesis,2017,vol. 49,# 14,p. 3149 - 3156]

611-97-2
238 suppliers
$21.00/25g

93318-88-8
13 suppliers
inquiry

874-60-2
399 suppliers
$10.00/5g

93318-88-8
13 suppliers
inquiry