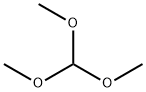
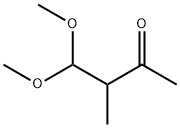
1,1-dimethoxypentan-3-one synthesis
- Product Name:1,1-dimethoxypentan-3-one
- CAS Number:31199-03-8
- Molecular formula:C7H14O3
- Molecular Weight:146.18
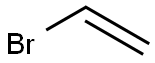
593-60-2
144 suppliers
$25.00/25g

124-41-4
731 suppliers
$12.00/25g
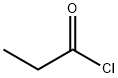
79-03-8
408 suppliers
$9.00/1g

31199-03-8
9 suppliers
inquiry
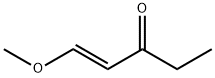
56279-28-8
0 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:propionyl chloride with aluminum (III) chloride in chloroform for 1 h;Cooling with ice;
Stage #2:Vinyl bromide in chloroform at 14; for 1 h;Cooling with ice;
Stage #3:sodium methylate in methanol at 20; for 18 h;Cooling with ice;Overall yield = 61.4 g;
Steps:
H 1,1-Dimethoxypentan-3-on
In a multi-neck flask with internal thermometer and KPG-stirrer 0.96 mol (89 g) propionic acid chloride were charged and cooled with a salt/ice freezing mixture. 0.82 mol (110 g) aluminium chloride was added portion wise and the mixture was stirred vigorously for 10 min and mixed with 50 ml of chloroform. Then, within about 1 h 0.93 mol (100 g) vinyl bromide was added in small portions (maximum internal temperature 14° C.). The mixture was stirred on ice for 1 h, and then the reaction mixture was poured onto 500 g ice and extracted several times with a total of 1 l of chloroform. The combined organic phases were washed 4 times with 1 l of water, dried with sodium sulphate and the chloroform was distilled off on a rotary evaporator. The residue was distilled in a rotary evaporator at 80° C. water bath temperature and 16 mbar (head temperature about 47° C.), which resulted in 118 g intermediate product (unstable, storage -18° C.). [0228] 118 g intermediate product were dissolved in 600 ml anhydrous methanol and cooled on ice. 1.03 mol (55.65 g) natrium methoxid were dissolved in 360 ml anhydrous methanol and added dropwise within 30 min and the mixture was stirred at room temperature for further 18 h. The resulting salt was filtered off, washed with a small amount of dry methanol and the filtrate was concentrated on a rotary evaporator. The residue was distilled at a rotary evaporator at a water bath temperature of 75° C. and 4 mbar (head temperature 40-52° C.). 61.4 g of a product mixture containing 62% (m/m) of the title compound (corresponding to 38 g) were obtained. [0229] 1H-NMR (DMSO-d6, 400 MHz): δ [ppm]=4.74 (t, 1H), 3.23 (s, 6H), 2.70 (d, 2H), 2.45 (q, 2H), 0.90 (t, 3H) (title compound, 62% m/m in product mixture); δ=7.69 (d, 1H), 5.61 (d, 1H), 3.69 (s, 3H), 2.48 (q, 2H), 0.96 (t, 3H) ((1E)-1-methoxypent-1-en-3-on, 38% in product mixture).
References:
Vifor (International) AG;Bark, Thomas;Buhr, Wilm;Burckhardt, Susanna;Burgert, Michael;Canclini, Camillo;Dürrenberger, Franz;Funk, Felix;Geisser, Peter Otto;Kalogerakis, Aris;Mayer, Simona;Philipp, Erik;Reim, Stefan;Sieber, Diana;Schmitt, Jörg;Schwarz, Katrin US2014/162994, 2014, A1 Location in patent:Paragraph 0227-0229
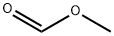
107-31-3
355 suppliers
$16.00/25mL
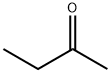
78-93-3
0 suppliers
$30.80/500ml

31199-03-8
9 suppliers
inquiry
36075-03-3
7 suppliers
inquiry