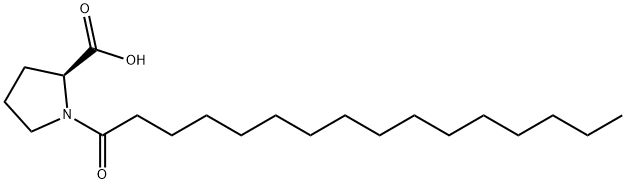
1-(1-Oxohexadecyl)-L-proline synthesis
- Product Name:1-(1-Oxohexadecyl)-L-proline
- CAS Number:59441-32-6
- Molecular formula:C21H39NO3
- Molecular Weight:353.54
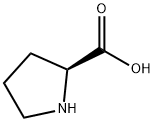
147-85-3
982 suppliers
$6.00/25g
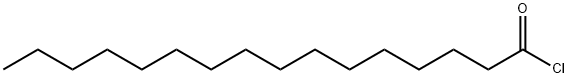
112-67-4
262 suppliers
$10.00/5mL

59441-32-6
38 suppliers
$90.00/100mg
Yield: 95%
Reaction Conditions:
with sodium hydroxide in 2-methyltetrahydrofuran;water at 0 - 20; for 4.5 h;
Steps:
A1 Molecule 3: Product Obtained by the Reaction Between Proline and Palmitoyl Chloride.
A solution of palmitoyl chloride (33 mL, 109.14 mmol) in methyl-THF (138 mL) is added dropwise to a solution of L-proline (25.13 g, 218.29 mmol) in a mixture of water (121.5 mL) and 10 N NaOH (27.3 mL, 272.86 mmol) at 0° C. under vigorous stirring while maintaining the temperature of the reaction medium 5° C. The reaction medium is stirred at from 4° C. to 20° C. for 1.5 h, then for 3 h at room temperature. After cooling down to 0° C., the pH is adjusted to 1.5 with concentrated hydrochloric acid (18.2 mL). The mixture is warmed to 20° C. and the phases are separated. The organic phase is washed with a 5% aqueous solution of KHSO4 (3×100 mL), water (100 mL) and then concentrated under reduced pressure. The residue is then recrystallized from heptane (200 mL). A solid of molecule 3 is obtained. Yield: 36.6 g (95%) 1H NMR (CDClhd 3, ppm): 0.87 (3H); 1.15-1.41 (24H); 1.57-1.74 (2H); 1.86-2.13 (3H); 2.35 (2H); 2.41-2.53 (1H); 3.39-3.52 (1H); 3.52-3.65 (1H); 4.37-4.44 (0.05H); 4.54-4.64 (0.95H); 7.83 (1H).
References:
ADOCIA US2021/244796, 2021, A1 Location in patent:Paragraph 0903; 0909-0911
14464-31-4
101 suppliers
$16.00/250mg

147-85-3
982 suppliers
$6.00/25g

59441-32-6
38 suppliers
$90.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

147-85-3
982 suppliers
$6.00/25g

59441-32-6
38 suppliers
$90.00/100mg

57-10-3
552 suppliers
$5.00/250mg

59441-32-6
38 suppliers
$90.00/100mg