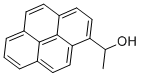
1-(1-PYRENYL)ETHANOL synthesis
- Product Name:1-(1-PYRENYL)ETHANOL
- CAS Number:65954-42-9
- Molecular formula:C18H14O
- Molecular Weight:246.3
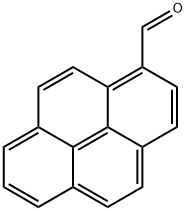
3029-19-4
190 suppliers
$15.00/1g

75-16-1
270 suppliers
$12.00/10ml

65954-42-9
8 suppliers
$650.00/500mg
Yield:65954-42-9 85%
Reaction Conditions:
in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Steps:
1-(1-Pyrenyl)ethanol (1e)
To a solution of 1-pyrenaldehyde (2.46 g, 10.7 mmol) in anhyd THF (30 mL) was added a 3 M solution of MeMgBr in THF (8 mL, 14 mmol) under N2. The reaction mixture was stirred at r.t. for 2 h. Then sat. aq NH4Cl (10 mL) was added and the resulting mixture was extracted with CH2Cl2 (2 × 30 mL). The combined organic layers were washed with brine (2 × 20 mL) and dried (anhyd MgSO4). After removal of the solvents at reduced pressure, the resulting solid was recrystallized from n-hexane to give 1e as white prisms in 85% yield (2.27 g, 9.15mmol). 1H NMR (CDCl3, 400 MHz): δ = 8.32 (d, J = 9.3 Hz, 1 H, ArH), 8.22 (d, J = 8.0 Hz, 1 H, ArH), 8.18 (d, J = 7.2 Hz, 3 H, ArH), 8.09 (d, J = 9.3 Hz, 1 H, ArH), 8.04 (s, 2 H, ArH), 8.0 (t, J = 7.6 Hz, 3 H, ArH), 5.96 (q, J = 6.4 Hz, 1 H, OCHCH3), 2.15 (s, 1 H, OH), 1.77 (d, J = 6.4 Hz, 1 H, CH3). 13C NMR (CDCl3, 101 MHz): δ = 139.07 (s), 131.37 (s), 130.64 (s), 130.62 (s) , 127.62 (CH), 127.48 (CH), 127.23 (s), 127.19 (CH), 125.94 (CH), 125.24 (CH), 125.12 (CH),125.03 (CH), 124.91 (s), 124.83 (s), 122.547 (CH), 122.40 (CH), 67.34 (CH), 25.08 (CH3). HRMS (EI): m/z calcd for C18H14O: 246.1045; found: 246.1040.
References:
Helberg, Julian;Marin-Luna, Marta;Zipse, Hendrik [Synthesis,2017,vol. 49,# 15,p. 3460 - 3470] Location in patent:supporting information

3264-21-9
109 suppliers
$37.00/1g

65954-42-9
8 suppliers
$650.00/500mg

3029-19-4
190 suppliers
$15.00/1g
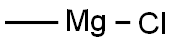
676-58-4
283 suppliers
$15.00/25ml

65954-42-9
8 suppliers
$650.00/500mg

129-00-0
326 suppliers
$9.00/1g

65954-42-9
8 suppliers
$650.00/500mg