
1-(1H-indazol-6-yl)ethanol synthesis
- Product Name:1-(1H-indazol-6-yl)ethanol
- CAS Number:181820-44-0
- Molecular formula:C9H10N2O
- Molecular Weight:162.1885
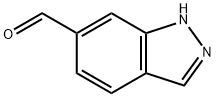
669050-69-5
121 suppliers
$23.00/250mg

75-16-1
265 suppliers
$12.00/10ml

181820-44-0
10 suppliers
inquiry
Yield:181820-44-0 60%
Reaction Conditions:
in tetrahydrofuran at -78 - 20;
Steps:
A51.A
To a solution of lH-indazole-6-carbaldehyde (200 mg, 1.37 mmol) in THF (7 mL) at -780C was added methyl magnesium bromide (1.4 mL, 4.11 mmol) dropwise under argon. The solution was warmed to rt and quenched with saturated ammonium chloride (2 mL). The mixture was extracted into ethyl acetate (3 x 10 mL), dried over MgSO4 and concentrated. The yellow oil was purified by silica gel chromatography (EtO Ac/Hex 4:1) to yield the title compound as a clear oil (135 mg, 60%). 1H NMR (400 MHz, CDCl3) δ 8.07 (s, IH), 7.76 (s, IH), 7.50-7.47 (m, 2H), 5.05 (q, 1 H, J = 7.4 Hz), 1.57 (d, 3H, J = 7.2 Hz); MS ESI [M + H]+, calcd for [C9H10N2O +H]+ 163.1; found m/z 163.0.
References:
WO2009/79767,2009,A1 Location in patent:Page/Page column 127