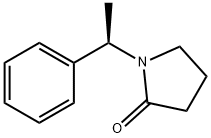
1-[(1R)-1-Phenylethyl]pyrrolidin-2-one synthesis
- Product Name:1-[(1R)-1-Phenylethyl]pyrrolidin-2-one
- CAS Number:60737-24-8
- Molecular formula:C12H15NO
- Molecular Weight:189.25
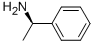
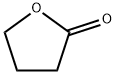
3886-69-9
560 suppliers
$14.00/25G
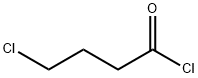
4635-59-0
463 suppliers
$17.67/10gm:
![1-[(1R)-1-Phenylethyl]pyrrolidin-2-one](/CAS/GIF/60737-24-8.gif)
60737-24-8
7 suppliers
inquiry
Yield:60737-24-8 99%
Reaction Conditions:
Stage #1: (R)-1-phenyl-ethyl-amine;4-Chlorobutanoyl chloridewith sodium hydroxide in dichloromethane;water at 0 - 10; for 0.25 h;
Stage #2: with N-benzyl-N,N,N-triethylammonium chloride in dichloromethane;water; for 2 h;Heating / reflux;
Steps:
4.1
R- (+)-OC-METHYLBENZYLAMINE (100 g, 825 mmol), methylene chloride (330 mL), and sodium hydroxide (26 g, 907 mmol) in water (412 mL) were introduced into a 3 liter 3-necked round bottom flask which was mechanically stirred and equipped with a 250 mL dropping funnel and thermometer. The mixture was cooled in an ice bath while stirring vigorously. 4-CHLOROBUTYRYL chloride (92 mL, 825 mmol) in dichloromethane (80 mL) was added dropwise keeping the temperature under 10 °C. The reaction mixture was stirred vigorously for 15 minutes, and transferred to a 2 liter separatory funnel. The dichloromethane layer was transferred into a 2 liter 3-necked round bottom flask containing benzyltriethylammonium chloride (9. 4 g, 41 mmol) to which a 50% solution of sodium hydroxide in water (330 mL) was added rapidly. The mixture was stirred vigorously then heated at reflux for 2 hours. Water was added and the dichloromethane layer was drawn off. The aqueous layer was back extracted 2x and the combined organic layers were washed with 1N HCI followed by water. The organic layer was dried over magnesium sulfate, filtered and concentrated to give the title compound (Yield: 155 g, 99%) which was used in the next step without further purification
References:
WO2005/26154,2005,A1 Location in patent:Page/Page column 97-98