
1,2,3,4-Tetrahydro-isoquinolin-6-ol Hydrochloride synthesis
- Product Name:1,2,3,4-Tetrahydro-isoquinolin-6-ol Hydrochloride
- CAS Number:63905-73-7
- Molecular formula:C9H12ClNO
- Molecular Weight:185.6507
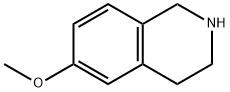
42923-77-3
143 suppliers
$21.00/100mg

63905-73-7
28 suppliers
inquiry
Yield:63905-73-7 90%
Reaction Conditions:
with hydrogenchloride;sodium hydrogencarbonate;aluminium trichloride in dimethylsulfide;dichloromethane;ethanol hydrochloride;ethyl acetate;
Steps:
R.138 6-Hydroxy-1,2,3,4-tetrahydroisoquinoline hydrochloride
Referential Example 138 6-Hydroxy-1,2,3,4-tetrahydroisoquinoline hydrochloride In dimethylsulfide (20 ml), 6-methoxy-1,2,3,4-tetrahydroisoquinoline (7.75 g) was dissolved, followed by the addition of aluminum chloride (19.0 g) under ice cooling. The resulting mixture was stirred at room temperature for 3 hours. Dichloromethane and dilute hydrochloric acid were added to the reaction mixture. A saturated aqueous solution of sodium bicarbonate was added to the water layer so separated to make it weakly alkaline, followed by the extraction with dichloromethane. After drying over anhydrous sodium sulfate, the solvent was then distilled off under reduced pressure. The residue was dissolved in saturated ethanol hydrochloride (100 ml) and the solvent was distilled off under reduced pressure. Ethyl acetate was added to the residue and the solid so precipitated was collected by filtration, whereby the title compound (7.91 g, 90%) was obtained. 1H-NMR (DMSO-d6) δ: 3.06(2H,t,J=5.9 Hz), 3.43(2H,m), 4.25(2H,s), 6.76(1H,d,J=2.0 Hz), 6.83(1H,dd,J=8.3,2.0 Hz), 7.15(1H,d,J=8.3 Hz), 9.71(3H,br s). MS (FAB) m/z: 150 (M+H)+.
References:
US6525042,2003,B1

50-00-0
845 suppliers
$10.00/25g
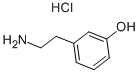
3458-98-8
80 suppliers
$50.00/100mg

63905-73-7
28 suppliers
inquiry