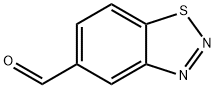
1,2,3-Benzothiadiazole-5-carboxaldehyde synthesis
- Product Name:1,2,3-Benzothiadiazole-5-carboxaldehyde
- CAS Number:394223-15-5
- Molecular formula:C7H4N2OS
- Molecular Weight:164.18
![benzo[d][1,2,3]thiadiazol-5-ylmethanol](/CAS2/GIF/615568-10-0.gif)
615568-10-0
14 suppliers
inquiry

394223-15-5
54 suppliers
$70.68/250mgs:
Yield:-
Reaction Conditions:
with manganese(IV) oxide
Steps:
23
Example 23 1-({4-[(1,2,3-benzothiadiazol-5-ylmethyl)amino]-1-piperidinyl}methyl)-9-fluoro-1-hydroxy-1,2-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-4-one Dihydrochloride A solution of 1-[(4-amino-1-piperidinyl)methyl]-9-fluoro-1-hydroxy-1,2-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-4-one (racemic) (48 mg, 0.15 mmol) and 1,2,3-benzothiadiazole-5-carbaldehyde (prepared by manganese (IV) oxide oxidation of benzo[1,2,3]thiadiazol-5-yl-methanol, for a synthesis see WO2003087098 Example 6(a)) (25 mg, 0.15 mmol) in dichloromethane (3 ml) and methanol (1 ml) was treated with sodium triacetoxyborohydride (95 mg, 0.45 mmol) for 5 hours at room temperature. Excess sodium bicarbonate solution was added and the mixture evaporated to dryness. The residue was chromatographed on silica gel, eluting with 0-50% methanol in dichloromethane, affording the free base as an oil (25 mg, 36%).MS (+ve ion electrospray) m/z 466 (MH+).δH (CDCl3, 250 MHz) 1.51 (2H, m), 1.95-2.25 (m, signals partly obscured by a water peak), 2.38 (1H, t), 2.55 (1H, t), 2.65 (2H, m), 2.85 (1H, d), 3.0 (2H, br.d), 3.36 (1H, d), 4.05 (2H, s), 4.40 (2H, m), 6.62 (1H, d), 6.90 (1H, t), 7.50 (1H, q), 7.70 (2H, m), 8.05 (1H, d), 8.60 (1H, s).The free base in methanol, was converted to the dihydrochloride salt by adding an excess of 1M hydrogen chloride in ether, followed by evaporation to dryness, to give a solid (30 mg).
References:
US2008/221110,2008,A1 Location in patent:Page/Page column 29
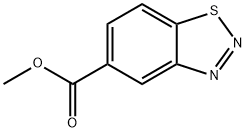
23616-15-1
51 suppliers
$65.00/50mg

394223-15-5
54 suppliers
$70.68/250mgs: