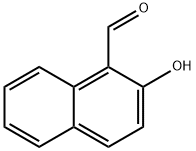
1-(2,3-dihydro-1H-perimidin-2-yl)-2-naphthol synthesis
- Product Name:1-(2,3-dihydro-1H-perimidin-2-yl)-2-naphthol
- CAS Number:307330-28-5
- Molecular formula:C21H16N2O
- Molecular Weight:312.36
Yield:307330-28-5 60%
Reaction Conditions:
in ethanol at 70; for 4 h;
Steps:
1-(2,3-dihydro-1h-perimidin-2-yl)naphthalen-2-ol (1)
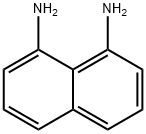
To a solution of naphthalene-1,8-diamine (632 mg, 4 mmol) in ethanol (30 mL) was added 2-hydroxy-1-naphthaldehyde(688 mg, 4 mmol), and the reaction mixture was heated at 70 °C for 4 h. Subsequently, it was cooled to room temperature. The resultant orange solution was filtered and a yellow precipitate was obtained. The precipitate was purified by recrystallization from ethanol/ethyl acetate (2:1) to give 749 mg of compound 1 as a 60% solid. M.p.: 145-147 °C. 1H NMR (300 MHz, CDCl3): δ 9.69 (s, 1H), 7.97 (d,J = 8.6 Hz, 1H), 7.84-7.80 (m, 2H), 7.49 (t, J = 7.1 Hz, 1H),7.37-7.29 (m, 5H), 7.2 (d, J = 8.9 Hz, 1H), 6.66 (dd,J1 = 6.7 Hz, J2 = 1.2 Hz, 2H), 6.45 (s, 1H), 4.72 (s, 2H). ESIMS: m/z = 312.97 [M+H]+; 646.82 [2M+Na]+.
References:
Ge, Yanning;Zhang, Dehua;Zhang, Xiaoyan;Liu, Yang;Du, Longfei;Wang, Yingying [Journal of Chemical Research,2021,vol. 45,# 1-2,p. 125 - 129]