
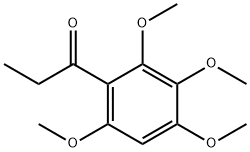
1-(2,3-Dihydroxy-4,6-diMethoxyphenyl)-1-propanone synthesis
- Product Name:1-(2,3-Dihydroxy-4,6-diMethoxyphenyl)-1-propanone
- CAS Number:94190-89-3
- Molecular formula:C11H14O5
- Molecular Weight:226.23
89880-48-8
1 suppliers
inquiry

94190-89-3
6 suppliers
$1320.00/250mg
Yield:94190-89-3 85%
Reaction Conditions:
with boron trichloride in dichloromethane at 0 - 20; for 20 h;regioselective reaction;
Steps:
1-(2-Hydroxy-4,6-dimethoxy-3-methoxymethoxyphenyl) propan-1-one (5)
To a stirred suspension of compound 10 (0.850 g, 3.34 mmol) was dissolved in dry CH2Cl2 (5 mL) at 0 oC. A 1N BCl3 solution in CH2Cl2 (13.0 mL) was added and the reaction mixture was allowed to warm up to room temperature and was left at room temperature for 20 h. Subsequently, the reaction mixture was poured into 30 ml of water and 20 ml of CH2Cl2 were added. The resulting mixture was filtered over celite, the organic Phase was collected and the aqueous phase was extracted twice with 20 ml of CH2Cl2. The combined organic layers were washed with 30 mL of water and dried over anhydrous Na2SO4 and concentrated in vacuo, the residue was purified by column chromatography using (30% EtOAc/hexane) to afford the pure ketodiol (0.641 g, 85%) as a yellow solid.
References:
Yadav;Revathi;Reddy, B.V. Subba [Tetrahedron Letters,2017,vol. 58,# 42,p. 3943 - 3946] Location in patent:supporting information
![1-[2-Hydroxy-4,6-diMethoxy-3-(1-oxopropoxy)phenyl]-1-propanone](/CAS/GIF/94190-88-2.gif)
94190-88-2
7 suppliers
$1455.00/1g

94190-89-3
6 suppliers
$1320.00/250mg

500-99-2
272 suppliers
$10.00/1g

94190-89-3
6 suppliers
$1320.00/250mg

99-24-1
437 suppliers
$10.00/5g

94190-89-3
6 suppliers
$1320.00/250mg

94190-87-1
8 suppliers
$140.00/100mg

94190-89-3
6 suppliers
$1320.00/250mg