
1,2,3-TRIMETHYL-1H-INDOLE-5-CARBOXYLIC ACID synthesis
- Product Name:1,2,3-TRIMETHYL-1H-INDOLE-5-CARBOXYLIC ACID
- CAS Number:356773-97-2
- Molecular formula:C12H13NO2
- Molecular Weight:203.24
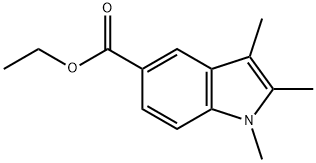
21523-64-8
0 suppliers
inquiry

356773-97-2
30 suppliers
$265.00/200mg
Yield:356773-97-2 84%
Reaction Conditions:
with water;sodium hydroxide in ethanol at 25 - 60; for 23 h;
Steps:
3 Example 3 - Preparation of 1,2,3-Trimethyl-1H-indole-5-carboxylic acid (Intermediate 3)
To a stirred solution of ethyl 2,3-Dimethyl-1H-indole-5-carboxylate (1.40 g, 6.44 mmol) in 5.00 mL DMF was added NaH (387 mg, 9.67 mmol, 60% in mineral oil) and MeI (2.74 g, 19.3 mmol, 3.0 eq.) at 0°C. The reaction mixture was stirred at 25°C for 2 h. TLC (petroleum Ether/ EtOAc = 5/1) check showed all starting materialRf = 0.40was consumed, a major new spot (Rf = 0.50) was observed. The reaction was diluted by 25.0 mL cold H2O, extracted with DCM (10.0 mL *3). The combined organic layers were dried over Na2SO4, concentrated to give desired product. Yield: 1.60 g (quant.). [00182] To a stirred solution of 2,3-Dimethyl-1H-indole-5-carboxylate (1.49 g, 6.44 mmol) in 20.0 mL EtOH and 5.00 mL H2O was added NaOH (773 mg, 19.3 mmol). The reaction mixture was stirred at 25°C for 16 h. TLC check (petroleum Ether/ EtOAc = 5/1) showed half of 2,3-Dimethyl-1H-indole-5-carboxylate still remained. The reaction was heated to 60oC for 7 h till full consumption of 2,3-Dimethyl-1H-indole-5-carboxylate by TLC check. The reaction was concentrated to remove most of the EtOH. The residue was diluted with 25.0 mL brine, acidified to pH = 3-4 and extracted with DCM (10.0 mL * 3). The organic phase was combined and dried over Na2SO4, concentrated to give desired product. Yield: 1.10 g (84%).
References:
WO2021/173593,2021,A1 Location in patent:Paragraph 00181-00182
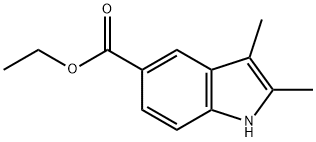
21523-62-6
45 suppliers
$65.00/100mg

356773-97-2
30 suppliers
$265.00/200mg