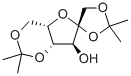
1,2,4,6-DI-O-ISOPROPYLIDENE-ALPHA-L-SORBOFURANOSE synthesis
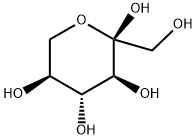
- Product Name:1,2,4,6-DI-O-ISOPROPYLIDENE-ALPHA-L-SORBOFURANOSE
- CAS Number:18604-19-8
- Molecular formula:C12H20O6
- Molecular Weight:260.28
Yield:18604-19-8 68.5%
Reaction Conditions:
with tin(ll) chloride in 1,2-dimethoxyethane at 70; for 2.3 h;Inert atmosphere;Temperature;Time;
Steps:
4.2. 1,2:4,6-di-O-isopropylidene-α-L-sorbofuranose (1)
To a suspension of L-sorbose (pulverized) (100.0 g, 0.56 mol) in 2,2-dimethoxypropane (300 mL, 3.0 vol) was added a solution of 1,2-dimethoxyethane(20 mL, 0.2 vol) containing SnCl2 (250 mg, 1.28 mmol).The mixture was refluxed gently with vigorous stirring under N2 at70 °C (bath temperature) for 2.3 h until other isomers of compound 1were carefully detected by TLC with ethyl acetate-petroleum ether (1:4,v/v) as a solvent. At this point, the reaction was quenched immediatelywith Et3N (1.26 mL) and filtered to remove the unreacted L-sorbose(10.0 g), and the solid was washed with CH2Cl2 (20 mL). The filtratewas concentrated to give a dark brown syrup. The syrup was thendissolved in CH2Cl2 (500 mL) and washed with water (100 mL) andbrine (100 mL×3). The CH2Cl2 fraction was dried over anhydrousNa2SO4, filtered, and evaporated to a brown syrup. The syrup was thendissolved with hot petroleum ether (360 mL) followed by filtration toremove the undissolved substance. Crystallization occurred when thehot petroleum ether extract was cooled slowly to room temperature.The precipitated product was collected by filtration, washed with cooln-hexane (200 mL), and dried in vacuum to afford 1 (89.0 g, 68.5%based on the recovered L-sorbose) as a pale yellow solid. M.p.71.3-73.7 °C, [α]D25=- 25.7° (c 1.010, acetone) [lit [6] M.p. 71-73 °C,[α]D25=- 24.7° (c 1.029, acetone)]; 1H NMR (600 MHz, DMSO-d6) δ5.22 (d, J=5.2 Hz, 1H), 4.11 (dd, J=3.4, 1.9 Hz, 1H), 4.01 (m, 1H),3.94 (s, 2H), 3.92 (dd, J=12.8, 3.3 Hz, 1H), 3.81 (dd, J=5.2, 1.9 Hz,1H), 3.67 (dd, J=12.8, 3.1 Hz, 1H), 1.40 (s, 3H), 1.34 (s, 3H), 1.32 (s,3H), 1.24 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 111.29, 110.64, 97.50,79.50, 74.36, 72.96, 72.58, 60.51, 28.64, 26.45, 25.41, 19.19; HRMS(ESI): m/z calcd for C12H20O6Na [M+Na]+ 283.1158, found 283.1167.
References:
Li, Jihui;Song, Xinjing;Wu, Tianxiao;Zhao, Liyu;Qin, Qiaohua;Cheng, Maosheng;Liu, Yang;Zhao, Dongmei [Carbohydrate Research,2019,vol. 480,p. 67 - 72]

7270-77-1
1 suppliers
inquiry

18604-19-8
5 suppliers
inquiry