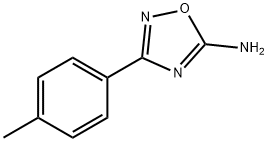
1,2,4-Oxadiazole, 3-(4-methylphenyl)- synthesis
- Product Name:1,2,4-Oxadiazole, 3-(4-methylphenyl)-
- CAS Number:16013-06-2
- Molecular formula:C9H8N2O
- Molecular Weight:160.17
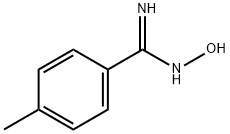
19227-13-5
100 suppliers
$10.00/250mg
149-73-5
523 suppliers
$12.00/5g

16013-06-2
9 suppliers
inquiry
Yield: 80%
Reaction Conditions:
with trifluoroacetic acid at 20 - 60; for 0.583333 h;
Steps:
3-(4-Methylphenyl)-1,2,4-oxadiazole (1u)37
Compound 1u was obtained according to the reported procedure.38 Toa solution of 4-methylbenzamide oxime (150 mg, 1.0 mmol) in trimethyl orthoformate (5 mL) was added a catalytic amount of trifluoroacetic acid at rt. After stirring for 5 min, the resulting solution was heated up to 60°C and stirred for additional 30 min. Then the mixture was concentrated and the residue was dissolved in EtOAc (3 × 10 mL). The combined organic layers were washed with brine (10 mL), dried to graphed on silica gel (eluent hexane-EtOAc, 2:1) to give oxadiazole1u; yield: 150 mg (80%); colorless oil. IR (KBr): 2925, 1640, 1545, 1525, 1340 cm-1. 1H NMR (400 MHz, CDCl3): = 8.75 (s, 1 H), 8.03 (d, J = 8.1 Hz, 2 H),7.33 (d, J = 8.1 Hz, 2 H), 2.45 (s, 3 H). 13C NMR (100 MHz, CDCl3): = 167.7, 164.6, 141.8, 129.6, 127.5,123.4, 21.5. HRMS (ESI-TOF): m/z [M + H]+ calcd for C9H9N2O: 161.0709; found:161.0703.
References:
Strelnikova, Julia O.;Rostovskii, Nikolai V.;Khoroshilova, Olesya V.;Khlebnikov, Alexander F.;Novikov, Mikhail S. [Synthesis,2021,vol. 53,# 2,p. 348 - 358]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

16013-06-2
9 suppliers
inquiry
36288-37-6
11 suppliers
inquiry

16013-06-2
9 suppliers
inquiry

19227-13-5
100 suppliers
$10.00/250mg
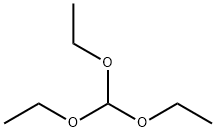
122-51-0
488 suppliers
$10.00/5ml

16013-06-2
9 suppliers
inquiry