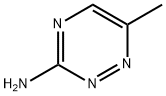
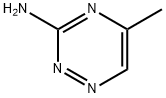
1,2,4-Triazin-3-amine,6-methyl-(9CI) synthesis
- Product Name:1,2,4-Triazin-3-amine,6-methyl-(9CI)
- CAS Number:18915-36-1
- Molecular formula:C4H6N4
- Molecular Weight:110.12
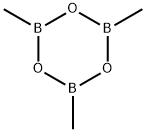
823-96-1
193 suppliers
$42.00/5G
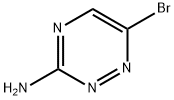
69249-22-5
131 suppliers
$12.00/250mg

18915-36-1
6 suppliers
inquiry
Yield:18915-36-1 70.2%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;caesium carbonate in 1,4-dioxane at 135; for 4 h;Inert atmosphere;Microwave irradiation;
Steps:
4B 4B. 6-Methyl-i ,2,4-triazin-3-amine
To solid 6-bromo-i,2,4-triazin-3-amine (0.419 g, 2.394 mmol) and cesium carbonate (2.80 g, 8.59 mmol) was added deoxygenated 1 ,4-dioxane (10 mE) and the mixture waspurged with a stream of nitrogen for 20 mm. To this mixture was added 2,4,6-trimethyl-i ,3,5,2,4,6-trioxatriborinane (0.420 g, 3.35 mmol) and Pd(dppf)C12.DCM (0.196 g, 0.239 mmol) and deoxygenation with a stream of nitrogen was continued for 10 mm. The mixture was then heated (micro-wave) in a sealed vial for 4 h at 135° C. The cooled mixture was evaporated and the resulting black residue was adsorbed on silica gel using only dichioromethane. Purification on an ISCO column (24 g; DCMlmethanol) gave the title com65 pound as a white solid (0.185 g, 1.680 mmol, 70.2%). ‘HNMR (400 MHz, CDC13) ? 8.08 (s, 1H), 5.12 (br s, 2H), 2.553H).
References:
US9598419,2017,B1 Location in patent:Page/Page column 44
2582-30-1
378 suppliers
$18.00/25g
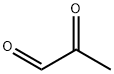
78-98-8
290 suppliers
$10.60/1gm:
6302-68-7
9 suppliers
inquiry

18915-36-1
6 suppliers
inquiry