
[1,2,4]Triazolo[1,5-a]pyridin-7-ol synthesis
- Product Name:[1,2,4]Triazolo[1,5-a]pyridin-7-ol
- CAS Number:1033810-70-6
- Molecular formula:C6H5N3O
- Molecular Weight:135.12
![7-Bromo[1,2,4]triazolo[1,5-a]pyridine](/CAS2/GIF/1053655-66-5.gif)
1053655-66-5
116 suppliers
$29.00/100mg
![[1,2,4]Triazolo[1,5-a]pyridin-7-ol](/CAS/20150408/GIF/1033810-70-6.gif)
1033810-70-6
115 suppliers
inquiry
Yield:1033810-70-6 40%
Reaction Conditions:
with lithium hydroxide monohydrate;potassium hydroxide;di-tertbutyl [2’,4’,6’-tris(propan-2-yl)-[1,1‘-biphenyl]-2-yl]phosphane in 1,4-dioxane;
Steps:
s23.1 Step 1: Synthesis of [1,2,4]triazolo[1,5-a]pyridin-7-ol.
To a solution of 7-bromo-[1,2,4]triazolo[1,5-a]pyridine (501.0 mg, 2.53 mmol) in 1,4-dioxane (8.0 mL)/H2O (2.0 mL) was added KOH (625.7 mg, 11.15 mmol), Pd2(dba)3 (263.1 mg, 0.28 mmol) and t-BuXPhos (231.1 mg, 0.54 mmol) at room temperature. The resulting mixture was stirred at 100°C for 4 h. After the reaction was completed, the mixture was cooled to room temperature and then extracted with EtOAc. The pH value of the aqueous layer was adjusted to pH 5 with 1N HCl. The mixture was evaporated in vacuo. The residue was purified by flash column chromatography with CH2Cl2/MeOH (5/1, v/v) to afford [1,2,4]triazolo[1,5-a]pyridin-7-ol (140.0 mg, 40%) as a white solid. LCMS (ESI, m/z): [M+H]+ =136.0.
References:
WO2022/6386,2022,A1 Location in patent:Paragraph 0434-0435
![7-(Benzyloxy)-[1,2,4]triazolo[1,5-a]pyridine](/CAS/GIF/1033810-72-8.gif)
1033810-72-8
5 suppliers
inquiry
![[1,2,4]Triazolo[1,5-a]pyridin-7-ol](/CAS/20150408/GIF/1033810-70-6.gif)
1033810-70-6
115 suppliers
inquiry
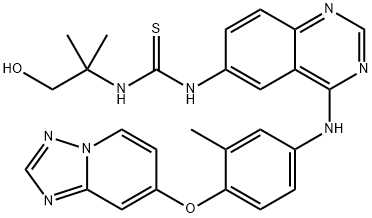
1429755-58-7
35 suppliers
inquiry
![[1,2,4]Triazolo[1,5-a]pyridin-7-ol](/CAS/20150408/GIF/1033810-70-6.gif)
1033810-70-6
115 suppliers
inquiry
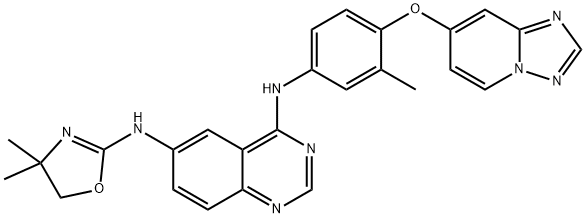
937263-43-9
179 suppliers
$90.00/10mg
![[1,2,4]Triazolo[1,5-a]pyridin-7-ol](/CAS/20150408/GIF/1033810-70-6.gif)
1033810-70-6
115 suppliers
inquiry
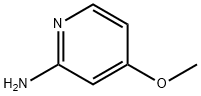
10201-73-7
261 suppliers
$8.00/1g
![[1,2,4]Triazolo[1,5-a]pyridin-7-ol](/CAS/20150408/GIF/1033810-70-6.gif)
1033810-70-6
115 suppliers
inquiry