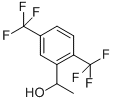
1-[2,5-BIS(TRIFLUOROMETHYL)PHENYL]ETHAN-1-OL synthesis
- Product Name:1-[2,5-BIS(TRIFLUOROMETHYL)PHENYL]ETHAN-1-OL
- CAS Number:364-47-6
- Molecular formula:C10H8F6O
- Molecular Weight:258.16
Yield:364-47-6 75%
Reaction Conditions:
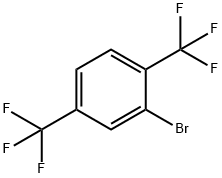
Stage #1: 2-bromo-1,4-bis-(trifluoromethyl)-benzenewith iodine;magnesium in tetrahydrofuran at 70; for 1 h;
Stage #2: acetaldehyde in tetrahydrofuran at -78 - 25; for 2 h;
Steps:
11.3 Step 3: l-(2,5-bis(trifluoromethyl)phenyl)ethanol (3):
To a mixture of Mg (2.1 g, 85 mmol) and iodine (63 mg, 0.25 mmol) in THF (80 mL) at 25 °C was added 2-bromo-l,4- bis(trifluoromethyl)benzene (25 g, 85 mmol). The mixture was warmed to 70 °C for 1 h to generate the Grignard reagent and then the solution was cooled to -78 °C. A solution of acetaldehyde (3.8 g, 85 mmol) in THF (80 mL) was added to the above solution and the reaction was warmed to 25 °C and stirred for 2 h. The residue was quenched by saturated NH4CI solution (100 mL) and water (300 mL), then extracted with ethyl acetate (200 mL x 2). The combined organic layers were washed with saturated brine (400 mL), dried over anhydrous NaiSCL, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (S1O2, Petroleum ether : Ethyl acetate = 1 :0 to 40: 1) to give 3 (17 g, 75 % yield) as a yellow oil. NMR (400 MHz, CDCh) d = 8.15 (s, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.64 (d, J = 8.4 Hz, 1H), 5.39-5.38 (m, 1H), 1.52 (d, J = 6.4 Hz, 3H).
References:
WO2020/242943,2020,A1 Location in patent:Paragraph 00233; 00235