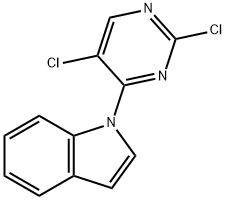
1-(2,5-DichloropyriMidin-4-yl)-1H-indole synthesis
- Product Name:1-(2,5-DichloropyriMidin-4-yl)-1H-indole
- CAS Number:293292-36-1
- Molecular formula:C12H7Cl2N3
- Molecular Weight:264.11
Yield:293292-36-1 26%
Reaction Conditions:
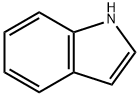
Stage #1: indolewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.5 h;
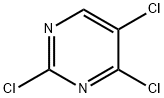
Stage #2: 2,4,5-trichloropyrimidine in N,N-dimethyl-formamide;mineral oil at 15; for 12 h;
Steps:
A95. 2,5-dichloro-4-indol-1-ylpyrimidine.
To a solution of indole (3.93 g, 33.56 mmol) in DMF (50 mL) was added NaH (60% in mineral oil,1.61 g, 40.27 mmol) portionwise at 0 °C. After stirring at 0 °C for 30 min, 2,4,5-triichloropyrimidine (5.00 g, 33.56 mmol) was added, then the mixture was allowed stirred at 15 °C for 12 hrs. The resulting mixture was quenched with water (300 mL) and the mixture was extracted with EtOAc (200 mL x 3), the combined organic layers were washed with water (300 mL x 4), dried with Na2SO4 and concentrated in vacuo. The residue was purified by silica gel chromatography (petroleum ether: EtOAc =10:1), to give 2,5-dichloro-4-indol-1-ylpyrimidine (2.00 g, 26%) as white solid.
References:
WO2017/120429,2017,A1 Location in patent:Page/Page column 315