
1-(2-Amino-6-fluorophenyl)ethanone synthesis
- Product Name:1-(2-Amino-6-fluorophenyl)ethanone
- CAS Number:869937-08-6
- Molecular formula:C8H8FNO
- Molecular Weight:153.15

75-16-1
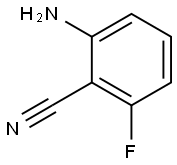
77326-36-4

869937-08-6
1. Methylmagnesium bromide (85.76 mL, 3.0 M ethyl ether solution) was added slowly and dropwise to a 20 mL THF solution of 2-amino-6-fluorobenzonitrile (5.0 g, 37 mmol) at -10°C. After the dropwise addition was completed, the reaction mixture was warmed to 70°C and stirred for 5 hours. Upon completion of the reaction, the reaction was quenched with 30 mL of 10% HCl solution and heated to reflux for 1 hour. Subsequently, the pH was adjusted to basic with NaHCO3 and extracted with EtOAc (30 mL x 3). The organic layers were combined, concentrated in vacuum and purified by silica gel column chromatography (eluent: PE/EtOAc = 100:1) to afford 1-(2-amino-6-fluorophenyl)ethanone (2.09 g, yield: 36.8%). 2. 1-(2-Amino-6-fluorophenyl)ethanone (2.0 g, 13.3 mmol) was dissolved in 35 mL of THF, DMAP (1.596 g, 16.6 mmol) and trichloroacetyl chloride (2.42 g, 13.3 mmol) were added, and the mixture was stirred at 0 °C. Subsequently, the reaction mixture was warmed to room temperature and stirred for 7 hours. After completion of the reaction, it was diluted with 50 mL of ice water and extracted with EtOAc (15 mL x 3). The organic layers were combined, concentrated in vacuum and purified by silica gel column chromatography (eluent: PE/EtOAc = 60:1) to afford Intermediate 4b (3.0 g, yield: 75.76%). Mass spectral data: m/z = 297 [M + H]+. 3. Intermediate 4b (1.4 g, 4.7 mmol) was dissolved in 10 mL of DMSO, ammonium acetate (1.3 g, 23 mmol) was added, and stirred at room temperature for 24 hours. After the reaction was completed, it was diluted with cold water, the precipitate was collected by filtration and dried under vacuum to give 5-fluoro-4-methyl-1H-quinazolin-2-one (0.8 g, yield: 95.6%). The product could be used in the next reaction without further purification. Mass spectral data: m/z = 179 [M + H]+. 4. 5-Fluoro-4-methyl-1H-quinazolin-2-one (300 mg, 1.68 mmol) was mixed with 3 mL of POCl3 and stirred at 80°C for 12 hours. After completion of the reaction, the mixture was poured into 20 mL of aqueous K2CO3 and extracted with EtOAc (5 mL x 3). The organic layers were combined, concentrated in vacuum and purified by silica gel column chromatography (eluent: PE/EtOAc = 50:1) to afford 2-chloro-5-fluoro-4-methyl-quinazoline (140 mg, yield: 42.5%) as a white solid. Mass spectral data: m/z = 197 [M + H]+.

75-16-1
274 suppliers
$12.00/10ml

77326-36-4
253 suppliers
$9.00/1g

869937-08-6
33 suppliers
$45.00/10mg
Yield:869937-08-6 36.8%
Reaction Conditions:
in tetrahydrofuran at -10 - 70; for 5 h;
Steps:
Synthesis of 2-chloro-5-fluoro-4-methyl-quinazoline
Synthesis of 2-chloro-5-fluoro-4-methyl-quinazoline To a solution of compound 4 (5.0 g,37 mmol) in 20 mL of THF was added dropwise MeMgBr (85.76 mL, 3.0 M in ether)at -10°C, then the mixture was stirred at70°C for 5 hours. The resulting mixture was quenched with 30 mL of 10 % HCI, heated to reflux for a hour, made alkaline by addition of NaHCO3 andextracted with EtOAc (30 mL χ 3), the organic layers were concentrated invacuum, the residue was purified by chromatography on silica gel (PE / EtOAc =100: 1 ) to afford compound 4a (2.09 g, yield: 36.8%).To a solution of the compound 4a (2.0g, 13.3 mmol) in 35 mL of THF was added DMAP (1 .596 g, 16.6 mmol) and CI3CCOCI (2.42 g, 13.3 mmol) at 0°C, then themixture was stirred at room temperature for 7 hours. The resulting mixture wasdiluted with ice water (50 mL), extracted with EtOAc (15 mL χ 3), the organiclayers were concentrated in vacuum, the residue was purified by chromatographyon silica gel (PE / EtOAc = 60: 1 ) to afford compound 4b (3.0 g, yield:75.76%). m/z = 297 [M + H]+ .To a solution of the compound 4b (1.4 g, 4.7 mmol) in 10 mL of DMSO was addedCH3COONH4 (1 .3 g, 23 mmol), the mixture was stirred at room temperature for 24hours. The resulting mixture was diluted with cold water, the formedprecipitate was collected by filtration and dried under vacuum to affordcompound 5-fluoro-4-methyl-1 H-quinazolin-2-one (0.8 g, yield: 95.6%), whichwas used for next step without further purification, m/z = 179 [M + H]+ .A mixture of 5-fluoro-4-methyl-1 H-quinazolin-2-one(300 mg, 1 .68 mmol) in 3 mL of POCI3 was stirred at 80°C for 12hours. Theresulting mixture was poured into 20 mL of aq. K2CO3 solution, extracted withEtOAc (5 mL χ 3), the organic layers wereconcentrated in vacuum, the residue was purified by chromatography on silicagel (PE / EtOAc = 50: 1 ) to give 2-chloro-5-fluoro-4-methyl-quinazoline (140mg, yield: 42.5%) as white solid. m/z = 197 [M + H]+ .
References:
WO2013/50527,2013,A1 Location in patent:Page/Page column 46; 47

434-76-4
333 suppliers
$12.00/10g
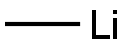
917-54-4
184 suppliers
$52.10/25ml

869937-08-6
33 suppliers
$45.00/10mg