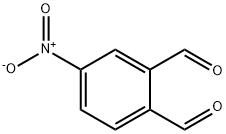
1,2-Benzenedicarboxaldehyde, 4-nitro- synthesis
- Product Name:1,2-Benzenedicarboxaldehyde, 4-nitro-
- CAS Number:13209-35-3
- Molecular formula:C8H5NO4
- Molecular Weight:179.13
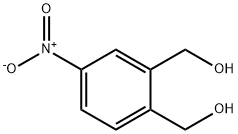
22162-19-2
9 suppliers
inquiry

13209-35-3
4 suppliers
inquiry
Yield:13209-35-3 52.1%
Reaction Conditions:
Stage #1: (4-nitro-1,2-phenylene)dimethanolwith oxalyl dichloride;dimethyl sulfoxide in tetrahydrofuran;dichloromethane at -78; for 2 h;
Stage #2: with triethylamine in tetrahydrofuran;dichloromethane at -78 - 20; for 0.666667 h;
Steps:
4-nitrophthalaldehyde
Dry dichloromethane (4 mL) was added to a previously flame dried round bottom flask. The mixture was cooled to -78° C., and oxalyl chloride (195 μl, 2.26 mmol) was added dropwise to the DCM with stirring. Next, dimethyl sulfoxide (250 μl 3.50 mmol) was added dropwise to the mixture at -78° C., and the mixture was stirred for 15 minutes. The previously prepared (4-nitro-1,2-phenylene)dimethanol dissolved in a DCM/THF mixture (2 mL, 3 DCM: 1 THF) was added dropwise and stirred at -78° C. for 2 hours. The flask containing was then rinsed with DCM/THF (2 mL, 3 DCM: 1 THF). Neat trimethylamine (2.6 mL, 18.51 mmol) was added to the stirring mixture and then allowed to stir for 10 minutes at -78° C., then allowed to warm to room temperature and then stirred for 30 minutes. The reaction mixture was then quenched with methanol (15 mL), stirred for 10 minutes and solvent was evaporated under reduced pressure yielding a glassy solid. The resulting crude product was purified using a dry loaded column packed with Merck grade silica with 60 Hexanes: 39 EtOAc: 1 MeOH. Pure fractions were collected, pooled and the identity of the compound was confirmed by NMR spectroscopy. Yield: 0.11 g, 52.1%. 1HNMR (methyl hemiacetal in equilibrium with phthalaldehyde) (300 MHz, CD3CN) δ 10.53 (s, 1H), 10.48 (s, 1H), 8.71 (d, J=2.3 Hz, 1H), 8.56 (dd, J=8.4, 2.3 Hz, 1H), 8.34-8.11 (m, 7H), 7.61 (m, 3H), 6.60-5.99 (m, 7H), 5.09 (m, 3H), 3.52-3.34 (m, 9H), 3.28 (s, 12H).
References:
US2021/24605,2021,A1 Location in patent:Paragraph 0118

6425-66-7
51 suppliers
$65.00/100mg

13209-35-3
4 suppliers
inquiry
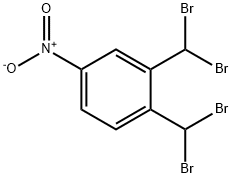
13209-16-0
39 suppliers
$45.00/50mg

13209-35-3
4 suppliers
inquiry
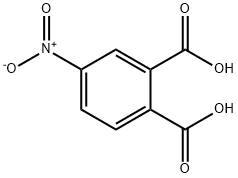
610-27-5
417 suppliers
$6.00/5g

13209-35-3
4 suppliers
inquiry
