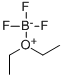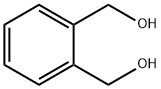
1,2-Benzenedimethanol synthesis
- Product Name:1,2-Benzenedimethanol
- CAS Number:612-14-6
- Molecular formula:C8H10O2
- Molecular Weight:138.16
Yield:612-14-6 95%
Reaction Conditions:
in tetrahydrofuran;diethyl ether;water
Steps:
5 EXAMPLE 5
EXAMPLE 5 A 2-liter, 4-necked glass reaction vessel equipped as described in Example 1 was charged with 44.6 grams (1.12 moles plus 5% excess) of sodium borohydride, 0.75 liter of tetrahydrofuran, and 0.185 liter (1.5 moles) of boron trifluoride diethyl etherate at 0°-5°C using the procedure described in Example 1. In a separate 3-liter, 4-necked glass reaction vessel equipped as described in Example 1 was placed 148 grams (1.0 mole) of phthalic anhydride and 0.25 liter of tetrahydrofuran. The resulting slurry was cooled to 0°-5°C using an external ice/water bath. The sodium borohydride-boron trifluoride reaction mixture was then transferred under nitrogen pressure to the anhydride/tetrahydrofuran slurry over a 1.5 hour period while maintaining a temperature of 5°-10°C. After stirring the reaction mixture for 12 hours at 20°C, 0.25 liter of water was added dropwise over a one hour period followed by one liter of diethyl ether. The aqueous layer was saturated with anhydrous potassium carbonate and the organic layer was removed. The aqueous layer was extracted with diethyl ether (2 * 0.25 liter) and the combined organic extracts were dried over anhydrous potassium carbonate, filtered, and concentrated on a rotary evaporator giving 131 grams (95% yield) of 1,2-benzene dimethanol as a crystalline solid, mp 58°-60°C.
References:
Aldrich-Boranes, Inc. US3978140, 1976, A
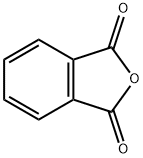
85-44-9
780 suppliers
$9.00/5g

612-14-6
185 suppliers
$10.00/1g
643-79-8
736 suppliers
$9.00/1g

612-14-6
185 suppliers
$10.00/1g
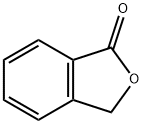
87-41-2
484 suppliers
$6.00/10g

612-14-6
185 suppliers
$10.00/1g
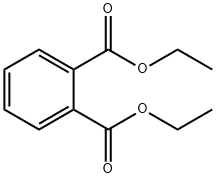
84-66-2
724 suppliers
$15.00/25mL

612-14-6
185 suppliers
$10.00/1g