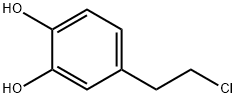
1,2-Benzenediol, 4-(2-chloroethyl)- synthesis
- Product Name:1,2-Benzenediol, 4-(2-chloroethyl)-
- CAS Number:104693-00-7
- Molecular formula:C8H9ClO2
- Molecular Weight:172.61
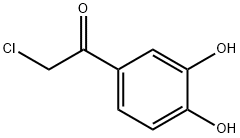
99-40-1
390 suppliers
$6.00/5g

104693-00-7
0 suppliers
inquiry
Yield:104693-00-7 96%
Reaction Conditions:
with triethylsilane;trifluoroacetic acid for 11 h;
Steps:
4-(2-chloroethyl)benzene-1,2-diol (3)
To a suspension of chloride 2 (80.42 g, 0.43 mol) intrifluoroacetic acid (165.40 mL, 2.15 mol) was added dropwise triethylsilane (276 mL, 2.15 mol) at 0°C, and the mixture was stirred at room temperature for 11 h. After completion of the reaction, mostof the volatiles were removed under reduced pressure, and the oily product was washed with boilingcyclohexane (10 × 500 mL). The cyclohexane layer was decanted and concentrated under reducedpressure to afford 71 g (96%) of compound 3 which was used for the next step without any furtherpurification. M.p.: 102-104 °C (EtOAc/n-hexane). 1H NMR (600 MHz, (CD3)2CO) δ: 7.75 (br s, 2, D2Oexch., 1-OH, 2-OH), 6.76 (d, 1, J = 8.2 Hz, H-6), 6.75 (s, 1, H-3), 6.60 (dd, 1, J = 8.0, 2.2 Hz, H-5), 3.70 (t,2, J = 7.1 Hz, CH2Cl), 2.90 (t, 2, J = 7.1 Hz, CH2CH2Cl). 13C NMR (151 MHz, (CD3)2CO) δ: 145.80 (C-2),144.67 (C-1), 130.87 (C-4), 121.06 (C-5), 116.74 (C-6), 116.06 (C-3), 46.10 (CH2Cl), 39.22 (CH2CH2Cl). (-)ESI QqToF (m/z): Calcd. for C8H8ClO2-: [M - H]- 171.0218, found 171.0224.
References:
Kalampaliki, Amalia D.;Giannouli, Vassiliki;Skaltsounis, Alexios-Leandros;Kostakis, Ioannis K. [Molecules,2019,vol. 24,# 18,art. no. 3239]
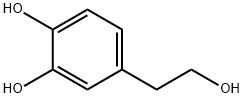
10597-60-1
519 suppliers
$28.00/100mg

104693-00-7
0 suppliers
inquiry
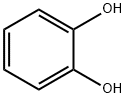
120-80-9
492 suppliers
$13.00/25g

104693-00-7
0 suppliers
inquiry