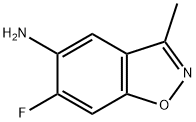
1,2-Benzisoxazol-5-amine,6-fluoro-3-methyl-(9CI) synthesis
- Product Name:1,2-Benzisoxazol-5-amine,6-fluoro-3-methyl-(9CI)
- CAS Number:221559-22-4
- Molecular formula:C8H7FN2O
- Molecular Weight:166.15

221559-21-3
1 suppliers
inquiry

221559-22-4
20 suppliers
inquiry
Yield:221559-22-4 55%
Reaction Conditions:
with hydrogenchloride;acetic acid;tin(ll) chloride in water at 40; for 2 h;Heating / reflux;
Steps:
1.4
Step 4: A solution of 4 (47 g, 240 mmol) in ACOH (800 ml) was warmed to 40° C. and to this warm solution was added a solution of SnCl2.H2O (150 g, 665 mmol) in conc. HCl (400 ml). The reaction mixture was refluxed for 2 h and then cooled to RT. The pH was carefully adjusted to 5-6 with aqueous NaOH to precipitate most of the tin salts and then ether was added to the mixture with constant stirring. After decanting the liquid, the organic layer was separated and the aqueous layer was back extracted several times with ether. The combined organic fractions were washed with brine, dried (Na2SO4), filtered and concentrated to give a brown oil. Purification by column chromatography (10-20% EtOAc in hexanes) gave 22 g (55% yield) of 5 as a yellow solid.
References:
US2007/72867,2007,A1 Location in patent:Page/Page column 5