1-(2-(benzyloxy)ethyl)-4-bromobenzene synthesis
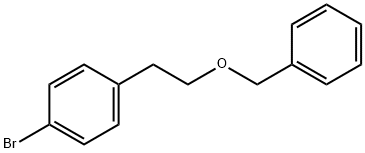
- Product Name:1-(2-(benzyloxy)ethyl)-4-bromobenzene
- CAS Number:170991-34-1
- Molecular formula:C15H15BrO
- Molecular Weight:291.18
Yield:170991-34-1 100%
Reaction Conditions:
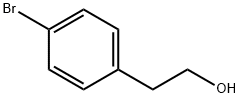
Stage #1: 4-bromophenethanolwith sodium hydride in DMF (N,N-dimethyl-formamide) at 0 - 20; for 1 h;
Stage #2: benzyl bromide in DMF (N,N-dimethyl-formamide) at 0 - 20; for 6 h;
Steps:
91.1 Example 91-1; Benzyl 2-(4-bromophenyl)ethyl ether
TO A SLURRY OF SODIUM HYDRIDE (abt. 60% oil suspension, 4.58g) in N, N-dimethylformamide (150ML) was added dropwise 2- (4-BROMOPHENYL) ethanol (20g) in N, N-dimethylformamide (50ML) at 0C, and the mixture was stirred for 1HR at room temperature. To the mixture was added dropwise benzyl bromide (19.6g) at 0C, and the mixture was stirred at room temperature for 6hrs. The resulting mixture was partitioned between saturated aqueous ammonium chloride and ethyl acetate. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, and concentrated in vacuo to give the title compound as a colorless oil (29. OG, 100%). 1H-NMR (300MHZ, CDCL3) : D 2.88 (2H, t, J=7Hz), 3.67 (2H, t, J=7Hz), 4.52 (2H, s), 7.11 (2H, d, J=8Hz), 7.27-7. 37 (5H, m), 7.41 (2H, d, J=8Hz).
References:
WO2004/65374,2004,A1 Location in patent:Page 99

100-39-0
423 suppliers
$10.00/10g

170991-34-1
5 suppliers
inquiry