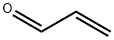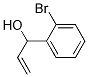
1-(2-broMophenyl)prop-2-en-1-ol synthesis
- Product Name:1-(2-broMophenyl)prop-2-en-1-ol
- CAS Number:114837-50-2
- Molecular formula:C9H9BrO
- Molecular Weight:213.07
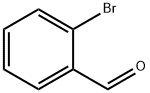
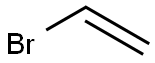
6630-33-7
404 suppliers
$9.00/10g

1730-25-2
195 suppliers
$66.20/100ml

114837-50-2
4 suppliers
inquiry
Yield:114837-50-2 91%
Reaction Conditions:
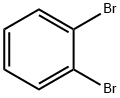
Stage #1: ortho-bromobenzaldehyde;allylmagnesium bromide in tetrahydrofuran at -18 - 20; for 17 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran;
Steps:
1
To a solution of allylmagnesium bromide (108 mL, 108 mmol, 2.0 eq) in THF was added a solution of 2-bromobenzaldehyde (10 g, 54 mmol, 1.0 eq) in THF (20 mL) dropwise at -180C. The mixture was allowed to reach room temperature for 17 h, then, quenched with saturated NH4Cl aqueous. The mixture was partitioned between brine and ethyl acetate. The organic layer was washed with brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography to provide l-(2-bromophenyl)prop-2-en-l-ol (10.5 g, 91%). LC-MS (m/z) = 213.0 [M + H]+.
References:
WO2011/19405,2011,A1 Location in patent:Page/Page column 207
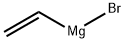
1826-67-1
208 suppliers
$60.89/100ml

6630-33-7
404 suppliers
$9.00/10g

114837-50-2
4 suppliers
inquiry
3536-96-7
195 suppliers
$67.00/100ml

6630-33-7
404 suppliers
$9.00/10g

114837-50-2
4 suppliers
inquiry