
1-(2-Chloro-6-hydroxyphenyl)ethanone synthesis
- Product Name:1-(2-Chloro-6-hydroxyphenyl)ethanone
- CAS Number:55736-04-4
- Molecular formula:C8H7ClO2
- Molecular Weight:170.59
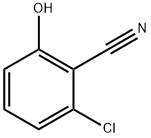
89999-90-6
91 suppliers
$16.00/1g

75-16-1
265 suppliers
$12.00/10ml

55736-04-4
79 suppliers
$8.00/100mg
Yield:55736-04-4 15%
Reaction Conditions:
Stage #1: 3-chloro-2-cyanophenol;methylmagnesium bromide in tetrahydrofuran;toluene at 20 - 65;Inert atmosphere;Sealed tube;
Stage #2: with hydrogenchloride in tetrahydrofuran;water;toluene;
Steps:
31.A; 32.A
Example 31; STEP A; To a solution of 2-chloro-6-hydroxybenzonitrile (614 mg, 4 mmol) in THF (10 mL) was added methylmagnesium bromide solution in toluene/THF (3/1, 1.4 M, 6.4 mL, 9 mmol). (Caution: Gas evolution.) The reaction mixture was stirred at room temperature under a flow of argon until gas evolution stopped. The reaction mixture was sealed in the tube, and heated to 65 0C for stirring overnight. The reaction mixture was quenched with water, and acidified with aqueous HCI solution. The organic mixture containing product was extracted with DCM. Column chromatography with an eluent of DCM/Hexane (1/1) gave product 31-1 (100 mg, 15% yield).; Example 32; STEP A; To a solution of 2-chloro-6-hydroxybenzonitrile (614 mg, 4 mmol) in THF (10 imL) was added methylmagnesium bromide solution in toluene/THF (3/1 , 1.4 M, 6.4 mL, 9 mmol). (Caution: Gas evolution.) The reaction mixture was stirred at room temperature under a flow of argon until gas evolution stopped. The reaction mixture was sealed in the tube, and heated to 65 °C for stirring overnight. The reaction mixture was quenched with water, and acidified with aqueous HCI solution. The organic mixture containing product was extracted with DCM. Column chromatography with an eluent of DCM/Hexane (1/1) gave product 31-1 (100 mg, 15% yield).
References:
WO2010/56631,2010,A1 Location in patent:Page/Page column 132-134
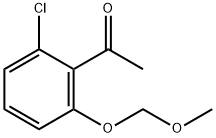
1241953-55-8
6 suppliers
inquiry

55736-04-4
79 suppliers
$8.00/100mg
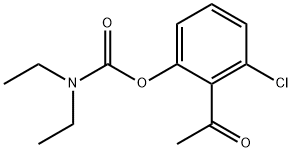
211449-30-8
0 suppliers
inquiry

55736-04-4
79 suppliers
$8.00/100mg
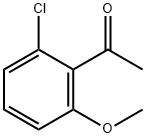
881883-32-5
59 suppliers
$13.00/250mg

55736-04-4
79 suppliers
$8.00/100mg
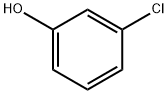
108-43-0
294 suppliers
$13.00/5g

55736-04-4
79 suppliers
$8.00/100mg