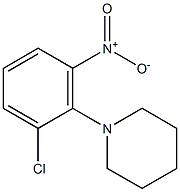
1-(2-chloro-6-nitrophenyl)piperidine synthesis
- Product Name:1-(2-chloro-6-nitrophenyl)piperidine
- CAS Number:3970-42-1
- Molecular formula:C11H13ClN2O2
- Molecular Weight:240.6861

110-89-4
3 suppliers
$15.96/100ML

3209-22-1
7 suppliers
$10.00/1g

3970-42-1
9 suppliers
$23.30/250mg
Yield:3970-42-1 320 mg (66%)
Steps:
8.a 5-Cyano-furan-2-carboxylic Acid (3-chloro-2-piperidin-1-yl-phenyl)-amide
a) 1-(2-Chloro-6-nitro-phenyl)-piperidine To a flask containing 2,3-dichloronitrobenzene (387 mg, 2.01 mmol) was added 3 mL of piperidine, and the result was heated to 80° C. overnight. The reaction was diluted with EtOAc (30 mL), washed with water (2*30 mL), dried (Na2SO4) and concentrated in vacuo. Purification by preparative tlc (20% EtOAc-hexane) yielded 320 mg (66%) of the title compound as a yellow solid. 1H-NMR (CDCl3; 400 MHz): δ 7.56-7.50 (m, 2H), 7.04 (t, 1H, J=8.1 Hz), 3.04 (br s, 4H), 1.69 (br s, 4H), 1.59 (br s obscured by water, 2H).
References:
US2005/131022,2005,A1

2106-49-2
178 suppliers
$5.00/5g

3970-42-1
9 suppliers
$23.30/250mg

110-89-4
3 suppliers
$15.96/100ML

2106-49-2
178 suppliers
$5.00/5g

3970-42-1
9 suppliers
$23.30/250mg