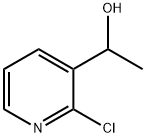
1-(2-chloropyridin-3-yl)ethanol synthesis
- Product Name:1-(2-chloropyridin-3-yl)ethanol
- CAS Number:131674-39-0
- Molecular formula:C7H8ClNO
- Molecular Weight:157.6
109-09-1
11 suppliers
$10.60/1gm:

75-07-0
420 suppliers
$14.00/5mL

131674-39-0
44 suppliers
$24.00/100mg
Yield:131674-39-0 64%
Reaction Conditions:
Stage #1: 2-chloropyridinewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -70; for 3 h;Inert atmosphere;
Stage #2: acetaldehyde in tetrahydrofuran;hexane at -70; for 3 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;hexane at -40;
Steps:
3.1
Step 1 : l-(2-Chloropyridin-3-yl)ethanol. Dry THF (400 mL) and n-butyllithium (1.6 M in hexane, 63 mL, 0.1 mol) were introduced into a IL flask under nitrogen at -70 0C. A solution of dry diisopropylamine (10.1 g, 0.1 mol) in THF (25 mL) was added dropwise to the mixture at -70 0C. The mixture was then kept for 1 h at 0 0C and then cooled to -70 0C. A solution of 2- chloropyridine (11.3 g, 0.1 mol) in THF (25 mL) was added dropwise to the mixture at - 70 0C and the mixture was stirred for 3 h at this temperature. A solution of acetaldehyde (4.85 g, 0.11 mol) in dry THF (50 mL) was then added dropwise and the mixture was kept for 3 h at -70 0C. A solution of water (4 mL) in THF (40 mL), acidified by a few drops of concentrated hydrochloric acid, was added to the mixture at -40 0C and then water (200 mL) was introduced at -10 0C. Extraction with diethyl ether (3x150 mL), drying over anhydrous sodium sulfate, and evaporation gave a crude product, which was purified by column chromatography to yield an oil (10 g, 64%). *1H NMR (CDCl3) 8.15 (dd, J = 5 and 2 Hz, 1 H), 7.95 (dd, J = 8 and 2 Hz, 1 H), 7.20 (dd, J = 8 and 5 Hz, 1 H), 5.15 (d, J = 7 Hz, 1 H), 3.90 (s, 1 H), 1.45 (d, J = 7 Hz, 3 H).
References:
WO2011/5759,2011,A2 Location in patent:Page/Page column 76-77

75-07-0
420 suppliers
$14.00/5mL

131674-39-0
44 suppliers
$24.00/100mg

75-16-1
269 suppliers
$12.00/10ml
36404-88-3
314 suppliers
$5.00/1g

131674-39-0
44 suppliers
$24.00/100mg