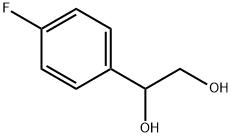
1,2-ETHANEDIOL, 1-(P-FLUOROPHENYL)- synthesis
- Product Name:1,2-ETHANEDIOL, 1-(P-FLUOROPHENYL)-
- CAS Number:17951-22-3
- Molecular formula:C8H9FO2
- Molecular Weight:156.15
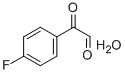
403-32-7
36 suppliers
$61.64/1g

17951-22-3
3 suppliers
inquiry
Yield:17951-22-3 100%
Reaction Conditions:
with sodium tetrahydroborate in ethanol; for 1 h;
Steps:
4.2.2. Synthesis of racemic 1-phenyl-1,2-ethanediol
General procedure: All racemic 1-phenyl-1,2-ethanediols were prepared in quantitative yields by the reduction of the corresponding substituted phenylglyoxal (1 mmol) with NaBH4 (2 mmol) in ethanol (7 mL) at 0 °C to room temperature for 1 h. After completion of the reaction the excess ethanol was removed. The reaction mixture was hydrolysed with dilute HCl and the mixture was extracted with ethyl acetate. The organic layer was dried, concentrated and purified by column chromatography.
References:
Mahajabeen, Pula;Chadha, Anju [Tetrahedron Asymmetry,2011,vol. 22,# 24,p. 2156 - 2160] Location in patent:experimental part
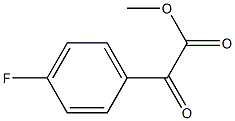
156276-23-2
20 suppliers
$165.00/0.5g

17951-22-3
3 suppliers
inquiry

766-98-3
245 suppliers
$6.00/1g

17951-22-3
3 suppliers
inquiry
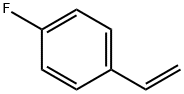
405-99-2
209 suppliers
$10.00/1g

17951-22-3
3 suppliers
inquiry
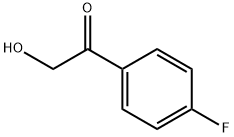
403-31-6
59 suppliers
$10.00/500mg

17951-22-3
3 suppliers
inquiry