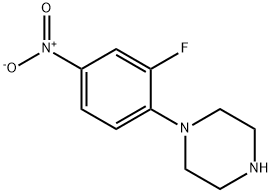
1-(2-Fluoro-4-nitrophenyl)piperazine synthesis
- Product Name:1-(2-Fluoro-4-nitrophenyl)piperazine
- CAS Number:154590-33-7
- Molecular formula:C10H12FN3O2
- Molecular Weight:225.22

154590-34-8
47 suppliers
$15.00/250mg

154590-33-7
91 suppliers
$19.10/250mg
Yield: 37%
Reaction Conditions:
Stage #1:4-(2-fluoro-4-nitrophenyl)-piperazine-1-carboxylic acid tert butyl ester with hydrogenchloride in ethyl acetate at 0 - 20; for 12 h;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate; pH=9
Steps:
52 Preparation of 1-(2-fluoro-4-nitrophenyl)piperazine
Reference Example 52 Preparation of 1-(2-fluoro-4-nitrophenyl)piperazine 4N Hydrogen chloride/ethyl acetate solution (10.0 ml, 40.0 mmol) was added to a solution of 4-(2-fluoro-4-nitrophenyl)piperazine-1-carboxylic acid tert-butyl ester (compound of Reference Example 14; 2.50 g, 7.68 mmol) in ethyl acetate (10 ml) under ice cooling, and the mixture was stirred at room temperature for 12 hours. The reaction mixture was adjusted to pH=9 by addition of saturated aqueous sodium hydrogencarbonate, and concentrated under reduced pressure. The obtained solid was collected by filtration and washed with water to give the title compound (0.563 g, 37%) as a yellow solid. 1H-NMR (CD3OD) δ: 2.98-3.05 (4H, m), 3.26-3.33 (4H, m), 7.12 (1H, t, J=9.0 Hz), 7.94 (1H, dd, J=13.5, 3.0 Hz), 8.02 (1H, ddd, J=9.0, 2.7, 0.9 Hz).
References:
INSTITUTE OF MEDICINAL MOLECULAR DESIGN. INC. US2008/227784, 2008, A1 Location in patent:Page/Page column 39
110-85-0
551 suppliers
$5.00/5 g

369-34-6
403 suppliers
$5.00/5g

154590-33-7
91 suppliers
$19.10/250mg