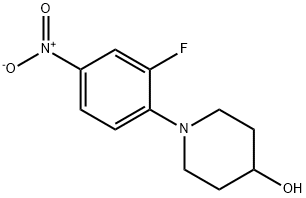
1-(2-fluoro-4-nitrophenyl)piperidin-4-o synthesis
- Product Name:1-(2-fluoro-4-nitrophenyl)piperidin-4-o
- CAS Number:873537-50-9
- Molecular formula:C11H13FN2O3
- Molecular Weight:240.23
Yield:873537-50-9 85%
Reaction Conditions:
with triethylamine in methanol at 50 - 60; for 24 h;
Steps:
2.3-6-1
3-6-1: Preparation of [3-fluoro-4-(4-hydroxypiperidino)]nitrobenzene To 100 ml of methanol were sequentially added 3 g (18.9 mmol) of 3,4-difluoronitrobenzene, 4.2 ml (30.2 mmol) of triethylamine and 2.79 ml (27.6 mmol) of 4-hydroxypiperidine, followed by reaction at a temperature of 50 to 60° for 24 hours. After the reaction was complete, the reaction liquid was cooled to room temperature. The resulting solid was filtered, washed with 20 ml of methanol and then dried under vacuum at about 40° to afford 5.13 g (yield: 85%) of the desired compound. Mass (M+): 241.1 1H-NMR (DMSO-d6): 1.51(m, 2H), 1.87(m, 2H), 3.06(m, 2H), 3.52(m, 2H), 3.81(m, 1H), 4.80(d, 1H), 7.14(t, 1H), 7.95(d, 1H), 7.98(s, 1H).
References:
US2011/306606,2011,A1 Location in patent:Page/Page column 15
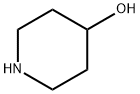
5382-16-1
13 suppliers
$9.00/5G

369-34-6
403 suppliers
$5.00/5g
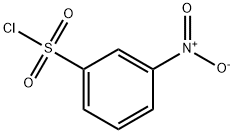
121-51-7
1 suppliers
$15.00/5G

873537-50-9
9 suppliers
inquiry