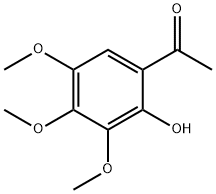
1-(2-Hydroxy-3,4,5-trimethoxyphenyl)ethanone synthesis
- Product Name:1-(2-Hydroxy-3,4,5-trimethoxyphenyl)ethanone
- CAS Number:30225-96-8
- Molecular formula:C11H14O5
- Molecular Weight:226.23
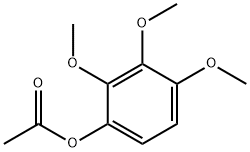
30225-80-0
0 suppliers
inquiry

30225-96-8
0 suppliers
inquiry
Yield:30225-96-8 690.1 mg
Reaction Conditions:
with boron trifluoride diethyl etherate at 100; for 0.5 h;Inert atmosphere;
Steps:
1-(2-Hydroxy-3,4,5-trimethoxyphenyl)ethanone (4)
To the crude 2,3,4-trimethoxyphenylacetate (1.2 g),BF3·OEt2 (4 mL, 31.8 mmol) was added at room temperatureunder Ar gas. After stirring for 30 min at 100 °C, thereaction mixture was poured into H2O, and the resultingmixture was extracted with EtOAc. The organic layer waswashed with saturated NaHCO3 aqueous solution, brine,and then dried over Na2SO4. Removal of the solvent gave a residue, which was purified by column chromatographyusing hexane-EtOAc (8:2, v/v) as the eluent to give 1-(2-hydroxy-3,4,5-trimethoxyphenyl)ethanone (4) (690.1 mg,68%) as a yellow solid. 1H-NMR (CDCl3; 400 MHz) δ12.44 (1H, s), 6.93 (1H, s), 4.04 (3H, s), 3.92 (3H, s), 3.86(3H, s), 2.59 (3H, s); 13C-NMR (CDCl3; 100 MHz) δ 203.1,153.1, 149.9, 145.0, 141.4, 114.2, 107.4, 61.3, 61.0, 59.7,26.7.
References:
Ishikawa, Kazuki;Takahashi, Kazunori;Hosoi, Syun;Takeda, Hisashi;Yoshida, Hinaka;Wakana, Daigo;Tsubuki, Masayoshi;Sato, Fumihiko;Tojo, Motoaki;Hosoe, Tomoo [Journal of Antibiotics,2019,vol. 72,# 2,p. 71 - 78]
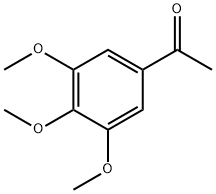
1136-86-3
152 suppliers
$9.00/1g
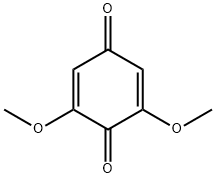
530-55-2
139 suppliers
$28.60/1g

17742-46-0
3 suppliers
inquiry
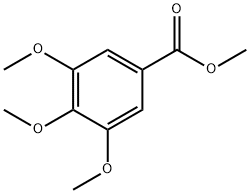
1916-07-0
430 suppliers
$6.00/25g

30225-96-8
0 suppliers
inquiry
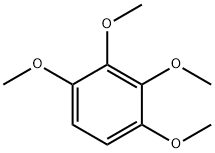
21450-56-6
20 suppliers
$121.00/100mg

75-36-5
568 suppliers
$17.92/100G

30225-96-8
0 suppliers
inquiry
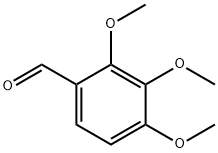
2103-57-3
506 suppliers
$9.00/10g

30225-96-8
0 suppliers
inquiry