
1,2-O-ISOPROPYLIDENE-3-BENZYLOXY-D-ALLOFURANOSE synthesis
- Product Name:1,2-O-ISOPROPYLIDENE-3-BENZYLOXY-D-ALLOFURANOSE
- CAS Number:57099-04-4
- Molecular formula:C16H22O6
- Molecular Weight:310.34
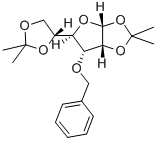
22331-21-1
75 suppliers
$70.00/10g

57099-04-4
19 suppliers
inquiry
Yield:57099-04-4 95%
Reaction Conditions:
with acetic acid in water at 20; for 72 h;
Steps:
1,2-Di-O-acetyl-3-O-benzyl-4-C-methanesulfonoxymethyl-5-O-methanesulfonyl-D-erythro-pentofuranose (12)
To acetic acid (2 L) and water (0.5 L) was added 3-O-benzyl-1,2:5,6-di-O-isopropylidene-α-D-allofuranose (500 g,1.43 mol) and the mixture was left to stand at room temperature for 72 h. The solution was then poured into an ice-coldsolution of sodium hydroxide (1.5 kg) in water (7.5 L) with vigorous stirring and addition of ice to keep the temperature below 20 °C. The mixture was then extracted with ethyl acetate (2 x 2.5 L) and the combined organics were washed with brine, dried over magnesium sulfate, combined with a second batch carried out on the same scale and concentrated under reduced pressure to give 3-O-benzyl-1,2-O-isopropylidene-α-D-allofuranose (921 g, 95%) as a yellow oil.
References:
Chapron, Christopher;Glen, Rebecca;La Colla, Massimiliano;Mayes, Benjamin A.;McCarville, Joseph F.;Moore, Stephen;Moussa, Adel;Sarkar, Ruhul;Seifer, Maria;Serra, Ilaria;Stewart, Alistair [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 12,p. 2699 - 2702]
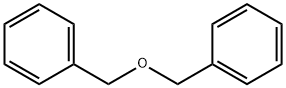
103-50-4
306 suppliers
$5.00/25g

57099-04-4
19 suppliers
inquiry
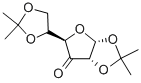
2847-00-9
89 suppliers
$105.00/1g

57099-04-4
19 suppliers
inquiry
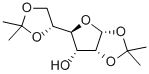
2595-05-3
257 suppliers
$7.00/1g

57099-04-4
19 suppliers
inquiry
582-52-5
465 suppliers
$5.00/5g

57099-04-4
19 suppliers
inquiry