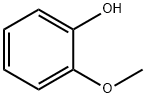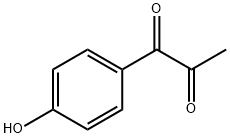
1,2-Propanedione,1-(4-hydroxyphenyl)- synthesis
- Product Name:1,2-Propanedione,1-(4-hydroxyphenyl)-
- CAS Number:10087-36-2
- Molecular formula:C9H8O3
- Molecular Weight:164.16
Yield:-
Reaction Conditions:
with formic acid;sodium formate in water at 110;
Steps:
C-O cleavage of different oxidized lignin model compounds
C-O cleavage of different oxidized lignin model compounds: (0279) Schemes 17 and 18 show that the approach described herein also function for other oxidized, lignin model compounds. Of particular note here is that all of the model compounds include a ketone in the benzyl position (i.e., the a-position) of the β-Ο-4 linkage. (0280) (0281) 0.2 mmol = 67 mg 3 equiv. (2 ι·ηΙ.: 100 μ1_) Conversion - 100% 92% (0282) (0283) 3: R=R"= H, R'= OH p-Hydroxyphenyl units (H) 89% 73% (0284) 4: R=H, R'= OH, R"= OMe guaiacyl units (G) 90% 84% (0285) 5: R=R"= OMe, R'= OH syringyl units (S) 940/,, 88% (0286) Scheme 18. C-0 Cleavage of various model compounds. (0287) As shown in Scheme 18, the specific yield of small, aromatic compounds was quite high. C-O cleavage of oxidized lignin to simple chemicals: (0288) Here, an authentic lignin sample was oxidized to introduce a benzylic-position ketone in at least a portion of the β-Ο-4 linkages, and then subjected to depolymerization via C-0 cleavage of the ether bond in the β-Ο-4 linkages. The resulting product mixture is shown in Fig. 8.
References:
WISCONSIN ALUMNI RESEARCH FOUNDATION;STAHL, Shannon, S.;COON, Joshua;RAHIMI, Alireza;ULBRICH, Arne WO2015/138563, 2015, A1 Location in patent:Page/Page column 35
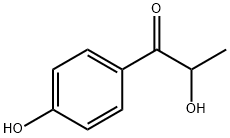
35263-54-8
0 suppliers
inquiry

10087-36-2
3 suppliers
inquiry