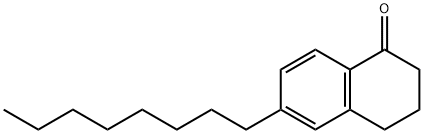
1(2H)-Naphthalenone, 3,4-dihydro-6-octyl- synthesis
- Product Name:1(2H)-Naphthalenone, 3,4-dihydro-6-octyl-
- CAS Number:945632-75-7
- Molecular formula:C18H26O
- Molecular Weight:258.4
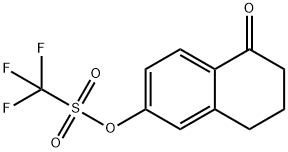
144464-64-2
9 suppliers
inquiry

945632-75-7
5 suppliers
inquiry
Yield: 82%
Reaction Conditions:
Stage #1:oct-1-ene with 9-bora-bicyclo[3.3.1]nonane in tetrahydrofuran at 20;
Stage #2:5-oxo-5,6,7,8-tetrahydronaphthalen-2-yl trifluoromethanesulfonate with potassium phosphate;potassium bromide;tetrakis(triphenylphosphine) palladium(0) in tetrahydrofuran;water at 65; for 2 h;
Steps:
2
Example 2; 6-Octyl-3.4-dihydro-2H-naphthalen-l-one (3); [00159] 9-BBN (0.5 M solution in THF, 20.2 mL, 10.1 mmol) was added to 1-octene (1.6 mL, 10.1 mmol) at room temperature. The solution was stirred, at room temperature, overnight. After this time, K3PO4 (2.93 g, 13.8 mmol), Pd(Ph3P)4 (191 mg, 0.17 mmol, 1.8 mol %), KBr (1.2 g, 10.1 mmol) and degassed H2O (0.18 mL, 10 mmol) were added. This was followed by a solution of compound 2 (2.7 g, 9.2 mmol) in dry degassed THF (10 mL). The reaction mixture was heated at 65 0C under argon for 2 hours. After cooling, the solution was acidified to pH 1 and extracted into EtOAc (100 mL). The aqueous layer was re-extracted with EtOAc (50 mL) and the combined organics washed with H2O (20 mL) and brine (40 mL). The organic layer was dried over magnesium sulfate and concentrated under vacuum. The residue was purified by column chromatography (Silica gel, 5% EtOAc in hexanes) to provide 1.93g of compound 3 (82%).
References:
UNIVERSITY OF VIRGINIA PATENT FOUNDATION WO2007/92638, 2007, A1 Location in patent:Page/Page column 30
3470-50-6
221 suppliers
$6.00/1g

945632-75-7
5 suppliers
inquiry

![9-Borabicyclo[3.3.1]nonane, 9-octyl-](/CAS/20210305/GIF/30089-00-0.gif)