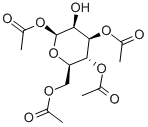
1,3,4,6-Tetra-O-acetyl-β-D-mannopyranose synthesis
- Product Name:1,3,4,6-Tetra-O-acetyl-β-D-mannopyranose
- CAS Number:18968-05-3
- Molecular formula:C14H20O10
- Molecular Weight:348.3
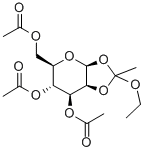
28140-37-6
40 suppliers
$140.00/1g

18968-05-3
121 suppliers
$28.00/250mg
Yield:18968-05-3 45%
Reaction Conditions:
with hydrogenchloride in water;acetone at 18 - 20; for 0.0833333 h;
Steps:
1,3,4,6-Tetra-O-acetyl-β-D-mannopyranose (5)
To a solution of the orthoester4(4.4 g, 12 mmol) in anhydrous acetone (27 mL) was added 1M hydrochloric acid (HCl) aq. (2.7 mL) in one portion with vigorous stirring (~18°C, water bath). After being stirred for an additional 5 min at room temperature, the reaction mixture was concentrated on a rotary evaporator below 20°C. The resulting solid was dissolved in CHCl3(25 mL) and washed with water (2 × 10 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated on a rotary evaporator. The residue was triturated with diethyl ether (Et2O) and recrystallized from EtOH to yield the tetraacetyl mannose5(1.9 g, 45%) as a white solid.Rfvalue 0.13 (hexane : EtOAc = 1 : 1). m.p. 134-136°C. HRMS (ESI):m/zfor C14H20NaO10([M+Na]+) cacld 371.09542, found 71.09581 (error 0.39 mmu, 1.06 ppm).
References:
Fadlan, Arif;Tanimoto, Hiroki;Ito, Tatsuya;Aritomi, Yusuke;Ueno, Maho;Tokuda, Masaya;Hirohara, Shiho;Obata, Makoto;Morimoto, Tsumoru;Kakiuchi, Kiyomi [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 8,p. 1848 - 1858] Location in patent:supporting information
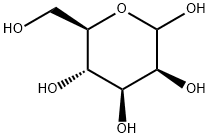
530-26-7
22 suppliers
inquiry
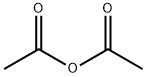
108-24-7
5 suppliers
$14.00/250ML

18968-05-3
121 suppliers
$28.00/250mg
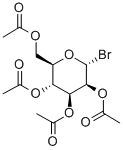
13242-53-0
57 suppliers
$100.00/5G

18968-05-3
121 suppliers
$28.00/250mg
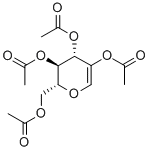
3366-47-0
42 suppliers
$90.00/500mg

18968-05-3
121 suppliers
$28.00/250mg
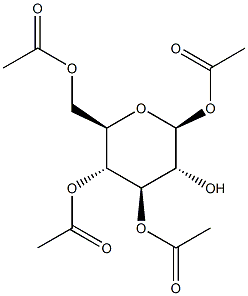
13036-15-2
8 suppliers
inquiry

18968-05-3
121 suppliers
$28.00/250mg