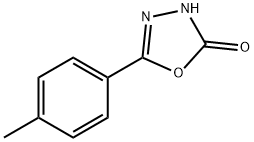
1,3,4-Oxadiazol-2(3H)-one, 5-(4-methylphenyl)- synthesis
- Product Name:1,3,4-Oxadiazol-2(3H)-one, 5-(4-methylphenyl)-
- CAS Number:83725-78-4
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
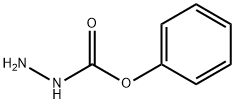
32315-10-9
412 suppliers
$10.00/1g
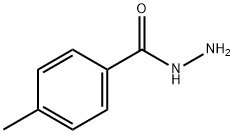
3619-22-5
109 suppliers
$12.00/5g

83725-78-4
12 suppliers
inquiry
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 1 h;Inert atmosphere;
Steps:
5.3.1. typical procedure for the synthesis of 1,3,4-oxadiazol-2(3H)-oneintermediates (18)
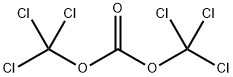
General procedure: The substituted benzoic acid 15 (6 mmol) was dissolved in 20 mL MeOH, SOCl2 (1.4 g, 12 mmol) was added slowly in ice-bath, then themixture was heated at 45 °C for 5 h. Upon completion, the mixture was concentrated, the residue was re-dissolved in EtOAc and washed with NaHCO3 and brine. The organic layer was dried with Na2SO4 and concentrated to give the desired ester intermediate 16, which was used in the next step without further purification.The above ester 16 was dissolved in 15 mL MeOH, hydrazine hydrate(2 mL, 32 mmol), then the mixture was heated at 45 °C for 5 h.Upon completion, the mixture was concentrated, the residue was redissolved in EtOAc and washed with brine. The organic layer was concentrated and purified by silica gel affording the desired acylhydrazine intermediate 17 as a white solid in good yield.Add benzhydrazide 17 (2 mmol), CH2Cl2 (20 mL) and DIPEA(0.52 g, 4 mmol) to a round bottomed flask under N2 protection.Triphosgene (0.3 g, 1 mmol) dissolved in DCM (4 mL) Using a syringe,the triphosgene/DCM solution was added dropwise to the stirred solutionof hydrazide, and stirred at room temperature for 1 h. The reactionmixture was concentrated by rotary evaporation, the crudeproduct was purified by chromatography on silica (DCM/MeOH=100:1) affording the desired products 18 as white solid (about70% yield for three steps). Example 18-D3, 1H NMR (600 MHz,DMSO-d6) δ 12.66 (s, 1H), 7.80 (d, J=8.6 Hz, 2H), 7.62 (d, J=8.6 Hz,2H). ESI-MS calcd for C8H4ClN2O2 [M-H]-, 195.0, found 195.2.
References:
Bi, Fangchao;Song, Di;Qin, Yinhui;Liu, Xingbang;Teng, Yuetai;Zhang, Na;Zhang, Panpan;Zhang, Nan;Ma, Shutao [Bioorganic and Medicinal Chemistry,2019,vol. 27,# 14,p. 3179 - 3193]

827-58-7
30 suppliers
$123.70/1gm:

83725-78-4
12 suppliers
inquiry

124-38-9
124 suppliers
$214.00/14L

3619-22-5
109 suppliers
$12.00/5g

83725-78-4
12 suppliers
inquiry