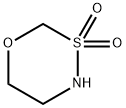
[1,3,4]OXATHIAZINANE 3,3-DIOXIDE synthesis
- Product Name:[1,3,4]OXATHIAZINANE 3,3-DIOXIDE
- CAS Number:863015-82-1
- Molecular formula:C3H7NO3S
- Molecular Weight:137.16
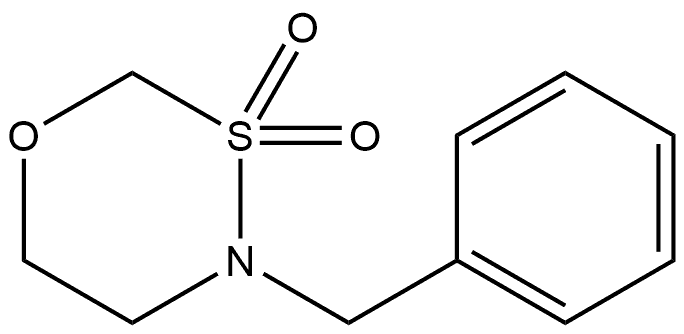
863015-81-0
0 suppliers
inquiry
![[1,3,4]OXATHIAZINANE 3,3-DIOXIDE](/CAS/GIF/863015-82-1.gif)
863015-82-1
27 suppliers
inquiry
Yield:863015-82-1 100%
Reaction Conditions:
with hydrogen;acetic acid in ethanol;ethyl acetate; under 3102.97 Torr; for 21 h;
Steps:
79
Example 79 [0239] To a solution of 4-benzyl-1 ,3,4-oxathiazinane 3,3-dioxide (1 .74 g, 7.66 mmol) in a mixture of ethyl acetate (50 mL) and absolute ethanol (50 mL) was added 1 mL of acetic acid. The solution was vacuumed and purged with argon for a minute and charged with 20% palladium(ll) hydroxide (100 mg). The reaction mixture was shaked on a hydrogenation apparatus under hydrogen pressure of 60 psi for 21 hrs, upon which TLC indicated the full conversion of starting material. The hydrogen source was removed and the reaction mixture was filtered via bed of celite. The filtrate was concentrated on rotavapor to dryness and the desired product was obtained as a white powder (1 .04 g, 100%). 1 H NMR (400 MHz,) δ (ppm): 7.16 (d, J = 2.0 Hz, 1 H), 4.65 (d, J = 4.4 Hz, 2H), 3.65 (d, J = 4.4 Hz, 2H), 3.29 (d, J = 4.4 Hz, 2H).
References:
WO2014/71298,2014,A1 Location in patent:Paragraph 0239

109-85-3
261 suppliers
$10.00/1g
![[1,3,4]OXATHIAZINANE 3,3-DIOXIDE](/CAS/GIF/863015-82-1.gif)
863015-82-1
27 suppliers
inquiry