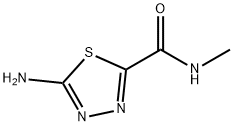
1,3,4-Thiadiazole-2-carboxamide, 5-amino-N-methyl- synthesis
- Product Name:1,3,4-Thiadiazole-2-carboxamide, 5-amino-N-methyl-
- CAS Number:1177423-60-7
- Molecular formula:C4H6N4OS
- Molecular Weight:158.18
64837-53-2
132 suppliers
$8.00/250mg

74-89-5
7 suppliers
$13.44/25ML

1177423-60-7
4 suppliers
inquiry
Yield:-
Reaction Conditions:
in methanol;ethanol at 20 - 70;
Steps:
45; 46
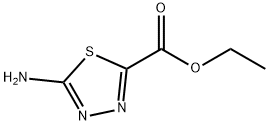
To a suspension of ethyl 5-amino-l,3,4-thiadiazole-2-carboxylate (106 mg, 0.612 mmol) in methanol (1.5 mL) was added 33 wt % ethanol solution of
References:
WO2009/100171,2009,A1 Location in patent:Page/Page column 105-106