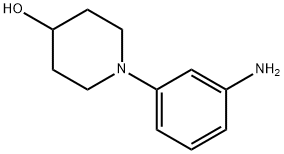
1-(3-AMINOPHENYL)PIPERIDIN-4-OL(WXC08112) synthesis
- Product Name:1-(3-AMINOPHENYL)PIPERIDIN-4-OL(WXC08112)
- CAS Number:1093107-38-0
- Molecular formula:C11H16N2O
- Molecular Weight:192.26
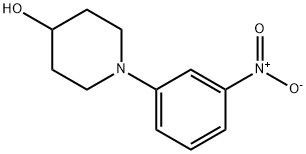
1093107-37-9
0 suppliers
inquiry

1093107-38-0
10 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol at 80; for 48 h;
Steps:
INTERMEDIATE 70 1 -(3 -Aminophenyl)piperidin-4-ol[00208] A solution of l-fluoro-3 -nitrobenzene (200 mg, 1.42 mmol), and piperidin-4-ol (430 mg, 4.25 mmol) in DMSO (1 mL) was heated at 80 0C for two days. The reaction mixture was diluted with water, extracted with EtOAc (two times), and the combined extracts were washed with brine, dried and concentrated. The residue was purified by flash chromatography (Siθ2,12g column, 0-100% EtOAc/DCM) to give an oil. The residue was dissolved in MeOH, and 10% Pd/C was added. The mixture was stirred under a hydrogen atmosphere (balloon) overnight. The catalyst was filtered off, and the filtrate was concentrated to give Intermediate 70 (400 mg, 146%) as a yellow oil. HPLC: Rt = 0.228 min. (CHROMOLITH column 4.6 x 50 mm eluting with 10-90% aqueous methanol over 4 min. containing 0.1% TFA, 4 mL/min., monitoring at 220 nm). MS (ES): m/z = 193 [M+H]+. Intermediate 70 was used in the synthesis of Example 154.
References:
WO2010/42699,2010,A1 Location in patent:Page/Page column 103-104