
1,3-Benzodioxol-5-amine, 4-methyl- synthesis
- Product Name:1,3-Benzodioxol-5-amine, 4-methyl-
- CAS Number:196091-24-4
- Molecular formula:C8H9NO2
- Molecular Weight:151.16
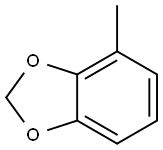
20487-10-9
10 suppliers
inquiry
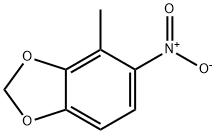
72744-52-6
0 suppliers
inquiry
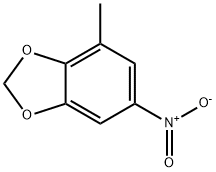
72744-53-7
0 suppliers
inquiry

196091-24-4
3 suppliers
inquiry
Yield:-
Reaction Conditions:
with nitric acid;palladium in methanol;chloroform;acetic anhydride;
Steps:
2 Example 2
A solution of 1.42 mL of concentrated nitric acid in 20 mL of acetic anhydride is added dropwise to a stirred solution of 2.90 g of 4-methyl-1,3-benzodioxole in 75 mL of acetic arthydride at -5° C. After 15 minutes the mixture is poured over 100 g of crushed ice and allowed to stir for 30 minutes as a yellow precipitate falls out of solution. The solid is filtered and taken up in chloroform, dried over magnesium sulfate and concentrated under reduced pressure to afford 2.85 g of an inseparable mixture of 5-nitro-4-methyl-1,3-benzodioxole and 6-nitro-4-methyl-1,3-benzodioxole. 5-Amino-4-methyl-1,3-benzodioxole. 2.85 g of the above mixture is mixed with 0.30 g of 5% palladium on carbon in 75 mL of methanol and placed under a 50 psi hydrogen atmosphere. The mixture is shaken for three hours at room temperature. The reaction mixture is filtered through Celite to yield a yellow solution. Concentration under reduced pressure affords an oil, which is purified by chromatography on silica gel to afford 0.62 g of 5-amino-4-methyl-1,3-benzodioxole. 5-Isothiocyanato-4-methyl-1,3-benzodioxole.
References:
US5663189,1997,A