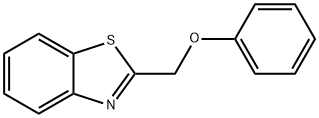
1,3-benzothiazol-2-ylmethyl phenyl ether synthesis
- Product Name:1,3-benzothiazol-2-ylmethyl phenyl ether
- CAS Number:37859-39-5
- Molecular formula:C14H11NOS
- Molecular Weight:241.31
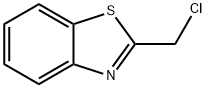
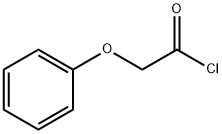
37859-43-1
115 suppliers
$12.00/250mg

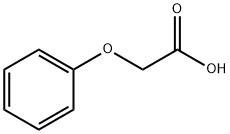
108-95-2
724 suppliers
$14.00/25g

37859-39-5
5 suppliers
inquiry
Yield:37859-39-5 56.02%
Reaction Conditions:
with potassium carbonate in acetone; for 4 h;Reflux;Williamson Ether Synthesis;
Steps:
3.1.2. General Procedure for the Synthesis of the Benzoxazole and Benzothiazole Derivatives(5a-m, 6a-m)
General procedure: To a solution of various substituted phenols (1 mmol) in dry acetone (30 mL) K2CO3 (1 mmol)and compound 3 or 4 (1 mmol) were added. After being stirred for 4 h at reflux temperature, thereaction mixture was cooled, filtered, and concentrated under vacuum. Then the residue was dilutedwith 30 mL ethyl acetate and sequentially washed with 30 mL 1 M HCl, aq. NaHCO3 solution andbrine in order. The organic layer was dried over MgSO4 and concentrated in vacuo. Purification of theresidue by chromatography on silica gel furnished target compounds. 1H-NMR, 13C-NMR and massspectroscopy (MS) of compounds 5a-m and 6a-m are shown in Supplementary Materials.
References:
Luo, Bo;Li, Ding;Zhang, An-Ling;Gao, Jin-Ming [Molecules,2018,vol. 23,# 10,art. no. 2457]
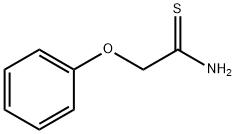
35370-80-0
27 suppliers
$44.20/250mg
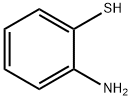
137-07-5
292 suppliers
$10.00/5g

37859-39-5
5 suppliers
inquiry