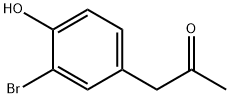
1-(3-Bromo-4-hydroxyphenyl)propan-2-one synthesis
- Product Name:1-(3-Bromo-4-hydroxyphenyl)propan-2-one
- CAS Number:655237-87-9
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07
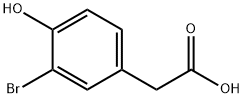
38692-80-7
115 suppliers
$13.00/1g
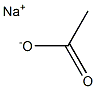
127-09-3
1080 suppliers
$8.00/100dtn(0.82g)

108-24-7
5 suppliers
$14.00/250ML

655237-87-9
9 suppliers
inquiry
Yield:655237-87-9 79%
Reaction Conditions:
Stage #1: 2-(3-bromo-4-hydroxyphenyl)acetic acid;sodium acetate;acetic anhydride for 21 h;Heating / reflux;
Stage #2: with sodium hydroxide;water pH=11; for 1 h;Heating / reflux;
Stage #3: with hydrogenchloride in water at 20; pH=6;
Steps:
1
Sodium acetate (50.5 g) was added to an acetic anhydride (100 ml) solution of 3-bromo-4-hydroxyphenylacetic acid (28.5 g). The mixture was refluxed for 21 hours with heating. The reaction mixture was returned to room temperature and was adjusted to pH 11 by adding a 20% aqueous sodium hydroxide solution, and then the mixture was refluxed for 1 hour with heating. The reaction mixture was returned to room temperature and was adjusted to pH 6 by adding a 10% aqueous hydrochloric acid solution. The whole was extracted with ethyl acetate. The organic layer was washed with water, a saturated aqueous sodium bicarbonate solution and saturated brine, and dried over anhydrous sodium sulfate. After filtration, the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate-n-hexane) to obtain 1-(3-bromo-4-hydroxyphenyl)acetone (22.2 g, yield = 79%).
References:
EP1783122,2007,A1 Location in patent:Page/Page column 19
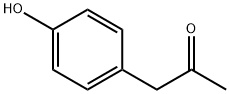
770-39-8
123 suppliers
$34.00/5g

655237-87-9
9 suppliers
inquiry

38692-80-7
115 suppliers
$13.00/1g

655237-87-9
9 suppliers
inquiry