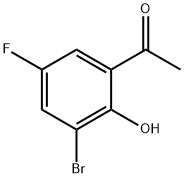
1-(3-Bromo-5-fluoro-2-hydroxy-phenyl)-ethanone synthesis
- Product Name:1-(3-Bromo-5-fluoro-2-hydroxy-phenyl)-ethanone
- CAS Number:393-62-4
- Molecular formula:C8H6BrFO2
- Molecular Weight:233.03
Yield:393-62-4 60%
Reaction Conditions:
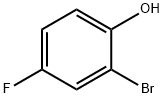
Stage #1: 2-bromo-4-fluoro-phenol;acetyl chloride at 50 - 55; for 1 h;
Stage #2: aluminum (III) chloride in dichloromethane at 10 - 135; for 2 h;
Steps:
Preparation of 1- (3-bromo-5-fluoro-2-hydroxyphenyl) ethanone; A mixture of 2-bromo-4-fluorophenol (64.0 kg, 1.00 mol equiv) and acetyl chloride (62.5 kg, 2.39 mol equiv) was stirred and heated at 50-55°C for one hour. Excess acetyl chloride (10. 8 kg) was removed by distillation and the residue was cooled to 25-30°C then diluted with dichloromethane (120 L). The mixture was further cooled to 10-15°C and aluminum chloride (51. 0 kg, 1.15 mol equiv) was added in two portions. The temperature of the mixture was raised to 130-135°C over one hour during which time dichloromethane (80 L) was removed by distillation. The mixture was maintained at 130-135°C for one hour, diluted with xylene (250 L) and cooled to 10-15°C. The reaction mixture was added to a solution of 30% w/w hydrochloric acid (25 L) in water (500 L). The layers were separated and the organic phase was extracted with 10% w/w sodium hydroxide solution (300 L). The aqueous extract was cooled to 10-15°C and adjusted to pH 6. 8-7. 2 with 30% w/w hydrochloric acid (55.0 kg). The solid was filtered off, washed with water (60 L), then with petroleum ether (100 L) and dried at 55-60°C under vacuum. The yield of 1- (3-bromo-5- fluoro-2-hydroxyphenyl) ethanone was 46.0 kg (60%).
References:
WO2005/90318,2005,A1 Location in patent:Page/Page column 21-22
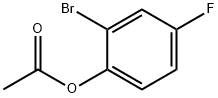
480439-44-9
10 suppliers
inquiry

393-62-4
17 suppliers
inquiry
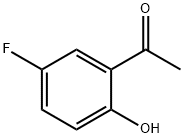
496-69-5
295 suppliers
$6.00/5g

393-62-4
17 suppliers
inquiry
394-32-1
354 suppliers
$9.00/5g

393-62-4
17 suppliers
inquiry
459-60-9
364 suppliers
$7.00/5g

393-62-4
17 suppliers
inquiry
